cobas® 8000 data manager
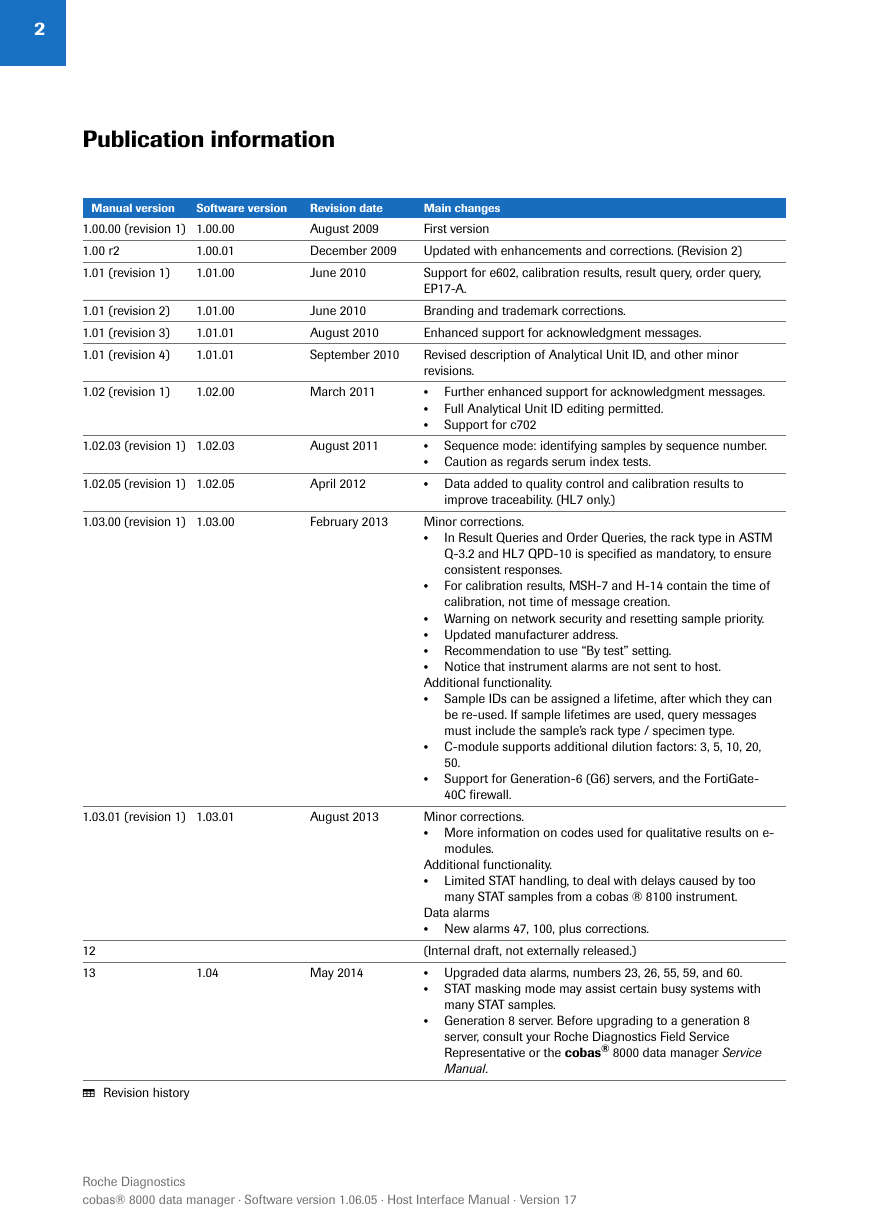
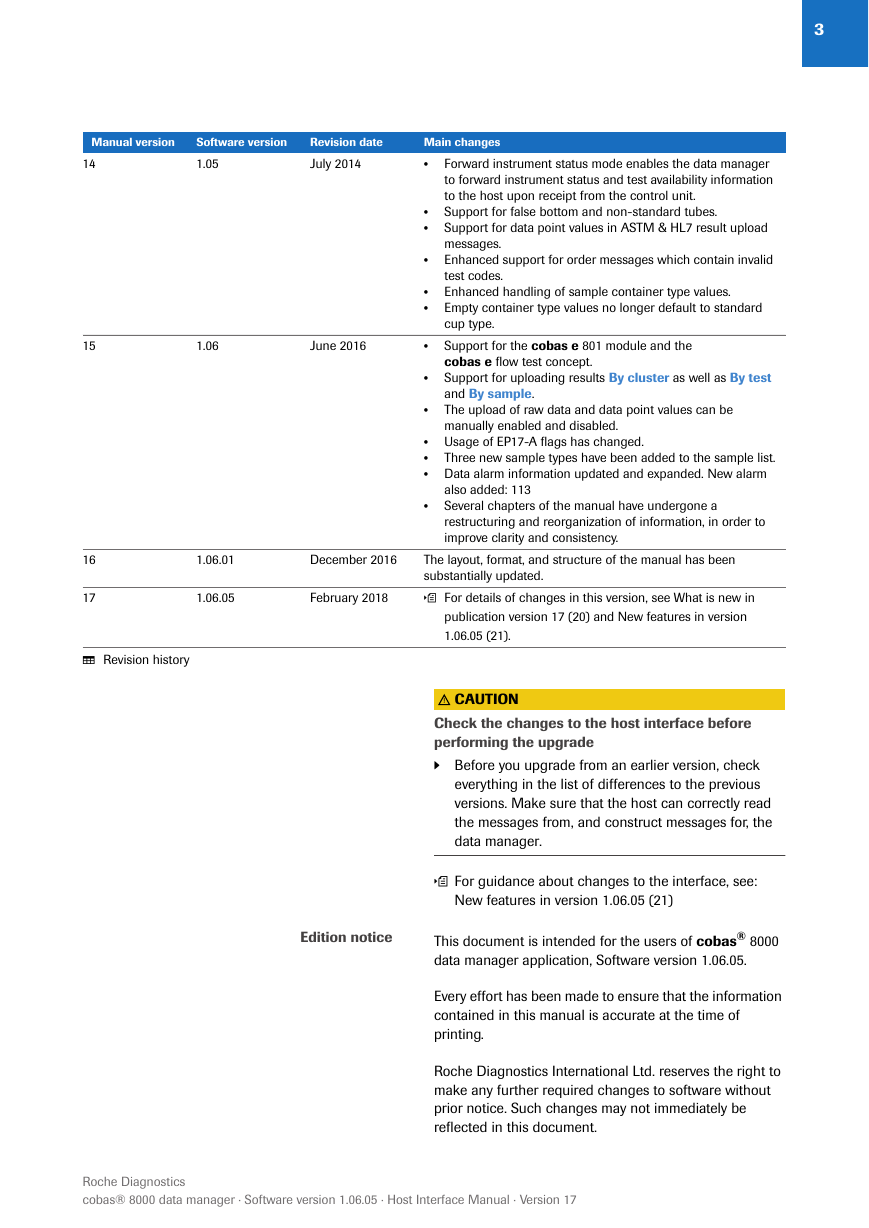
Publication information
Contact addresses
Table of contents
Intended use
Symbols and abbreviations
Safety information classification
Safety information
What is new in publication version 17
New features in version 1.06.05
Workflow overview
Overview
Purpose and scope
Who this manual is for
Content
System architecture
Overview of the system
Overview of modules
LIS communications
Text encoding
Sample processing
Supported sample types
Processing samples
Barcodes
Sample tubes and racks
Test selection
Sample container types
When received from the host
When received from the control unit
Repeat, rerun, and reflex tests
Batch and realtime processing
Batch and realtime and the data manager
Batch and realtime modes
Configuring the data manager for the host modes
Multiple test selection messages
Reusing sample IDs and sample lifetimes
Sample ID used with different sample types
Identifying samples on the instrument
Returning results to the host
Passing results automatically to the host
System time and result handling
EP17-A and the result abnormal flags
Result suppression for results outside the technical limit
About cobas e flow tests
Introducing the cobas e flow test
What is a cobas e flow test?
QC for cobas e flow tests
About repeat, rerun, and reflex tests with cobas e flow tests
Communication scenarios and message types
List of communication and message types
Communication scenarios
Realtime test selection download
Batch test selection download
Result query
Order query
Instrument status and test availability messages
Other message scenarios
Details of message types
Query message
Sending test selections
Sending patient test results
Raw data and data points in patient test results
Result query
Order query
Order and result queries with limited sample lifetime
QC results
Calculated QC results
Calibration data results
Masking
Instrument status and test availability
Message flow
Message flow batch download from host
Message flow for batch download, upload by sample
Message flow for TS Inquiry from instrument / data manager
Configuration
Configuring the data manager
Configuring the data manager
The host connection
Starting and stopping the host connection
Using the data manager without a host connection
Configuring the data manager host interface options
Identifying the data manager application to the host
Test selection inquiry timeout settings
Test selection inquiry message settings
Connection protocol settings
HL7 acknowledgment handling settings
Throttle instrument settings
Pass-through mode
Logging level settings
Rack and position mode
STAT masking mode
Forward instrument status mode (HL7 only)
Viewing result upload settings on the data manager
Enabling/disabling raw data and data point upload to the host
Identifier of the analytical unit sent to host
cobas e flow test-related settings
Enabling/disabling cobas e flow test subresult upload
Sending single QC results for calculated QC
Sending additional link information for calculated QC
Using legacy result status
Configuring omitted result reporting
Assigning result type for cobas e flow test result upload
Assigning host codes to cobas e flow test results and generated subresults
About host code assignment
Activating or deactivating the host code assignment for a cobas e flow test result
Activating or deactivating the host code assignment for a cobas e flow test generated subresult
Assigning host codes to cobas e flow test results
Assigning host codes to cobas e flow test generated subresults
Host code assignment example
Getting a trace file from the data manager
Physical layer specifications
Connecting a Generation 5 server
Connecting a Generation 6 server
Connecting a Generation 8 server
Network connection
Serial connection
Configuring the control unit
Enabling communication with data manager
Settings for communications with data manager
Text settings
Test selection inquiry settings
Test selection inquiry for rerun tests
Enabling requests for rerun or reflex tests
Result upload settings
Configuring result upload
Upload by test, sample, or cluster
Automatic rerun timing
When ordering HPE tests
Getting a trace file from the control unit
ASTM reference
ASTM protocol (LIS2 - A2)
Overview of the ASTM protocol
Communication processing layers
ASTM lower layer
ASTM message framing
ASTM syntax
Coding rules for the messages
Message transmission phases
Checksum calculation / message frame
ASTM text content (LIS2 - A2)
List of record levels
List of messages used in host communication
Messages transmitted by the data manager
Messages transmitted by the host
Description of ASTM records
Message Header Record
Patient Information Record
Order Record
Comment Record (following Order Record)
Result Record
Result Record description
R-4 description
R-4 for tests with quantitative or quantitative/qualitative results
R-4 for tests with qualitative or qualitative/quantitative results
R-4 for tests with data point and raw data values
About qualitative results
Comment Record (following Result Record)
Query Record (Request Information Record)
Photometric Calibration Result M(PCR)
ISE Calibration Result Record - M(ICR)
ISE data alarms
ISE calibration analytical data
E-module (immunology) Calibration Result Record - M(ECR)
Message Termination Record
ASTM communication examples
Low level trace file
Test selection inquiry from data manager
Routine test selection inquiry and download
STAT sample test selection inquiry and download
Test selection inquiry and download if no sample found
Test selection inquiry with sequence number
Regular test patient result upload messages
Realtime ISE result upload
ISE result upload - sample short
C-module result upload with no flag
C-module result with alarm flag
Batch upload of current results
Batch upload of current results - sample short
Batch upload all previous results
Result below normal range
Result below level of detection
Result with sample identified by sequence number
e 602 result with data point values
e 801 result message including raw data
e 801 result message with raw data and data points
cobas e flow test result upload messages
Result for sample with multiple cobas e flow tests and embedded tests
Result for cobas e flow test with raw data, non-reported embedded tests and generated subresult
cobas e flow test with qualitative and quantitative result
cobas e flow test calculated QC upload
Quality control uploads
Realtime quality control upload
Realtime quality control upload with a standby bottle
Batch quality control upload
Batch quality control upload with a standby bottle
Calibration result uploads
C-module calibration result upload
ISE-module calibration result upload
E-module calibration result upload
Result request from host and the data manager result upload
Request for currently active results
Request for all results
Result request but no results or sample not found
Result request with a sequence number
Result request with an expired sample
Order query from the host
Order query for all tests
Order query for all open tests
Order query but no open tests
Order query but sample not found
Order query with sample identified by sequence number
HL7 reference
HL7 protocol
Overview of HL7
Physical communication
HL7 text content
HL7 messages
Messages sent only by the data manager
Messages sent only by the host
Messages sent by either data manager or the host
HL7 segment description
Message Header Segment - MSH
Message Acknowledgment Segment - MSA
Patient Identification Segment - PID
Specimen Segment - SPM
SPM in test selections, patient results and quality control results
SPM in calibration results
Specimen Container Detail Segment - SAC
Observation Request Segment - OBR
Timing Quantity Segment - TQ1
Observation Result Segment - OBX
Observation Result Segment - OBX (for patient results)
Observation Result Segment - OBX (for QC results)
Observation Result Segment - OBX (for calibration results)
OBX-5 description
OBX-5 for patient and QC tests with quantitative or quantitative/qualitative results
OBX-5 for patient and QC tests with qualitative or qualitative/quantitative results
OBX-5 for patient and QC tests with data point and raw data results
About qualitative flags
Measurement of c-module photometric Calibration Results
Measurement of ISE Calibration Results
Measurement of e-module immunological Calibration Results
Calibration alarm flags
Test Code Detail Segment - TCD
Substance Identifier Segment - SID
Comment Segment - NTE
Types of comment segment
Query Parameter Segment - QPD (for a test selection inquiry)
Query Parameter Segment - QPD (for a Result Query)
Query Parameter Segment - QPD (for an Order Query)
Response Control Parameter Segment - RCP
Equipment Detail Segment - EQU (from host to data manager)
Equipment Command Segment - ECD
Equipment Detail Segment - EQU (from data manager to host)
Inventory Detail Segment - INV
HL7 acknowledgment handling
Acknowledgment flags in messages from the data manager
Inquiry for test selections
Result report patient
Result report quality control
Result report calibration data
Test selection upload from data manager to host
Acknowledgment message from data manager to host
Acknowledgment flags sent in response to host messages
Result query
Order query
Masking
Test selection download
Acknowledgment message
HL7 communication examples
Test selection inquiry and download
Test selection inquiry for routine rack
Test selection inquiry for STAT rack
Routine rack (AL) with acknowledgment
Routine rack invalid test (ER)
Test selection inquiry no sample found
Test selection inquiry with a sequence number
Regular test patient result upload messages
Single patient result upload
Batch upload all previous results
Batch upload all current results
Batch upload of current results sample short
Result outside user range
Result outside normal range
Quantitative test result
e 602 result message with data point values
cobas e flow test result upload messages
cobas e flow test EP17-A messages with flags
cobas e flow test result with EP17-A data alarm
Sample with multiple cobas e flow tests and embedded tests
cobas e flow test with qualitative and quantitative result
cobas e flow test with generated subresult
cobas e flow test with non-reported embedded tests
cobas e flow test with data alarms and non-reported embedded tests
cobas e flow test result with custom host codes
cobas e flow test result message with raw data and data points
Qualitative and cut-off index result messages
Qualitative/cut-off index result for regular test
Qualitative/cut-off index result for cobas e flow test
Embedded test result with qualitative result in cobas e flow test
Quality control result upload messages
Realtime QC from c-module
Realtime QC from c-module standby bottle
Batch QC from c-module
Batch QC from c-module standby bottle
QC results from e-module
Symmetric quality control result message
Asymmetric quality control result message
cobas e flow test calculated QC result messages
Calculated QC result message
Calculated QC result flagged with generic alarm
Calculated QC result with raw data
Calibration result upload messages
C-module calibration result
ISE-module calibration result
E-module calibration results
Masking messages
Result requests from the host
Request for all results
Request for final results
No results found
Sample not found
Result request with a sequence number
Result request with an expired sample
Order requests from the host
Request for all tests
Request for open tests
No tests found
Sample not found
Order request with a sequence number
Instrument status and test availability messages
Test availability request
Test availability update
Instrument status request
Instrument status update
Appendices
Data alarms
List of numerical code data alarms and output characters on the control unit
List of data alarms
Data alarms of ISE tests
ADC.E
Calc.?
Cal.E (sample flag)
Cal.I
ClcT.E
CmpT.?
CmpT.E
Edited
< >ISE
>I.H
>I.HI
>I.I
>I.L
>I.LH
>I.LHI
>I.LI
ISE.E
ISE.N
MIXLOW
na.LHI
Over.E
Reag.S
>Rept/
Samp.C
Samp.O
Samp.S
< >Test
>Test/
Data alarms of photometric tests
>Abs
ADC.E
Calc.?
Cal.E (sample flag)
Cal.I
ClcT.E
CmpT.?
CmpT.E
>Cuvet
Det.S
Edited
>I.H
>I.HI
>I.I
>I.L
>I.LH
>I.LHI
>I.LI
>Kin
>Lin
MIXLOW
MIXSTP
na.LHI
OBS.RM (c 702 module only)
OBS.RR
Over.E
>Proz
>React
ReagEx
Reag.S (c 701/c 702 modules only)
>Rept/
Samp.?
Samp.C
Samp.O
Samp.S
>Test/
Data alarms of immunology tests
ADC.E (e 801 module only)
Calc.? (e 602 module only)
Cal.E (sample flag)
Cal.I (e 602 module only)
CarOvr
Cell.T
Clot.E
>Curr
Curr.E
Edited
eFlow.E (e 801 module only)
eFlow.W (e 801 module only)
>I.H (e 602 module only)
>I.HI (e 602 module only)
>I.I (e 602 module only)
>I.L (e 602 module only)
>I.LH (e 602 module only)
>I.LHI (e 602 module only)
>I.LI (e 602 module only)
Inc.T
na.LHI (e 602 module only)
OBS.RR
Over.E
ReagEx
Reag.F
Reag.H
Reag.S
Reag.T
>Rept/
Samp.B
Samp.C
Samp.O
Samp.S
SLLD.E
SLLD.N
SysR.S
SysR.T
>Test/
WB.S
WBSS.T (e 801 module only)
WB.T
Alarms for calibrations
Cal.E
Cond.E
Diff.E
Duplicate error
Dup.E (cobas c modules)
Dup.E (cobas e modules)
IStd.E
Mono.E (e 602 module only)
Prep.E
Rsp1.E
Rsp2.E
S1A.E
SD.E
Sens.E
>Sig
Sig.E
Slop.E
Std.E
Std.E (ISE module)
Std.E (cobas c modules)
Sys.E
Data alarm for QC materials
QCErr
List of data alarms for QC measurements
Rerun test list
Alarm priorities
Instrument alarms
Instrument alarms
Result message codes for cobas e flow tests
Result message codes
Using MODULAR PRE-ANALYTICS
Sample IDs and barcodes
Handling query messages without barcodes
Configuring the control unit
Rerun without barcodes
Order of messages
Handling batch test selections with strict rack and position
Configuring data manager
Identifying the sample
Clearing the sample list
Identifying patient samples by sequence number
Identifying samples
Sample IDs and barcodes
Patient samples without barcodes
Identifying patient samples by sequence number
Sequence numbers and sample IDs
What the data manager understands
Important information for using sequence numbers
Using sample sequence numbers in ASTM
Test selection inquiry
Test selection download
Test result message
Using sample sequence numbers in HL7
Test selection inquiry
Test selection download
Test result message
List of configuration settings for barcode and sequence modes
Preparing the host communication
General differences in the ASTM protocol
Message encoding and transport
Sample identification
Sample types
Patient demographics
ASTM message types
HL7 message types
Differences in ASTM message definitions
General differences in the ASTM messages
Message Header Record
Message Termination Record
Patient Record
Order Record
Result Record
Comment Record (following an Order Record)
Comment Record (following a Result Record)
Query Record
Photometric Calibration Result Record
ISE Calibration Result Record
E-module (Immunology) Calibration Result Record
Other differences
Encoding
Rerun results
LIS communications
Identifying samples by sequence number
Traceability information for quality control and calibration results
The specimen segment SPM with quality control results
The specimen segment SPM with calibration results
The substance identifier segment SID
Data points
Text encoding tables
ASCII character codes
Western European characters in UTF-8
Glossary
Glossary
Index
Index














 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc