8536d_ch01_001-023 8/1/02 4:25 PM Page 1 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
Overview of the
Immune System
T
defense system that has evolved to protect animals
from invading pathogenic microorganisms and
cancer. It is able to generate an enormous variety of cells and
molecules capable of specifically recognizing and eliminat-
ing an apparently limitless variety of foreign invaders. These
cells and molecules act together in a dynamic network whose
complexity rivals that of the nervous system.
Functionally, an immune response can be divided into
two related activities—recognition and response. Immune
recognition is remarkable for its specificity. The immune
system is able to recognize subtle chemical differences that
distinguish one foreign pathogen from another. Further-
more, the system is able to discriminate between foreign
molecules and the body’s own cells and proteins. Once a for-
eign organism has been recognized, the immune system
recruits a variety of cells and molecules to mount an appro-
priate response, called an effector response, to eliminate or
neutralize the organism. In this way the system is able to
convert the initial recognition event into a variety of effector
responses, each uniquely suited for eliminating a particular
type of pathogen. Later exposure to the same foreign organ-
ism induces a memory response, characterized by a more
rapid and heightened immune reaction that serves to elimi-
nate the pathogen and prevent disease.
This chapter introduces the study of immunology from
an historical perspective and presents a broad overview of
the cells and molecules that compose the immune system,
along with the mechanisms they use to protect the body
against foreign invaders. Evidence for the presence of very
simple immune systems in certain invertebrate organisms
then gives an evolutionary perspective on the mammalian
immune system, which is the major subject of this book. El-
ements of the primitive immune system persist in verte-
brates as innate immunity along with a more highly evolved
system of specific responses termed adaptive immunity.
These two systems work in concert to provide a high degree
of protection for vertebrate species. Finally, in some circum-
stances, the immune system fails to act as protector because
of some deficiency in its components; at other times, it be-
comes an aggressor and turns its awesome powers against its
own host. In this introductory chapter, our description of
immunity is simplified to reveal the essential structures and
function of the immune system. Substantive discussions, ex-
perimental approaches, and in-depth definitions are left to
the chapters that follow.
chapter 1
Numerous T Lymphocytes Interacting with a Single
Macrophage
I Historical Perspective
I Innate Immunity
I Adaptive Immunity
I Comparative Immunity
I Immune Dysfunction and Its Consequences
Like the later chapters covering basic topics in immu-
nology, this one includes a section called “Clinical Focus”
that describes human disease and its relation to immunity.
These sections investigate the causes, consequences, or treat-
ments of diseases rooted in impaired or hyperactive immune
function.
Historical Perspective
The discipline of immunology grew out of the observation
that individuals who had recovered from certain infectious
diseases were thereafter protected from the disease. The
Latin term immunis, meaning “exempt,” is the source of the
English word immunity, meaning the state of protection
from infectious disease.
Perhaps the earliest written reference to the phenomenon
of immunity can be traced back to Thucydides, the great his-
torian of the Peloponnesian War. In describing a plague in
Athens, he wrote in 430 BC that only those who had recov-
ered from the plague could nurse the sick because they
would not contract the disease a second time. Although early
societies recognized the phenomenon of immunity, almost
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 2 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
2
P A R T I
Introduction
two thousand years passed before the concept was success-
fully converted into medically effective practice.
The first recorded attempts to induce immunity deliber-
ately were performed by the Chinese and Turks in the fif-
teenth century. Various reports suggest that the dried crusts
derived from smallpox pustules were either inhaled into the
nostrils or inserted into small cuts in the skin (a technique
called variolation). In 1718, Lady Mary Wortley Montagu, the
wife of the British ambassador to Constantinople, observed
the positive effects of variolation on the native population
and had the technique performed on her own children. The
method was significantly improved by the English physician
Edward Jenner, in 1798. Intrigued by the fact that milkmaids
who had contracted the mild disease cowpox were subse-
quently immune to smallpox, which is a disfiguring and of-
ten fatal disease, Jenner reasoned that introducing fluid from
a cowpox pustule into people (i.e., inoculating them) might
protect them from smallpox. To test this idea, he inoculated
an eight-year-old boy with fluid from a cowpox pustule and
later intentionally infected the child with smallpox. As pre-
dicted, the child did not develop smallpox.
Jenner’s technique of inoculating with cowpox to protect
against smallpox spread quickly throughout Europe. How-
ever, for many reasons, including a lack of obvious disease
targets and knowledge of their causes, it was nearly a hun-
dred years before this technique was applied to other dis-
eases. As so often happens in science, serendipity in
combination with astute observation led to the next major
advance in immunology, the induction of immunity to
cholera. Louis Pasteur had succeeded in growing the bac-
terium thought to cause fowl cholera in culture and then had
shown that chickens injected with the cultured bacterium de-
veloped cholera. After returning from a summer vacation, he
injected some chickens with an old culture. The chickens be-
came ill, but, to Pasteur’s surprise, they recovered. Pasteur
then grew a fresh culture of the bacterium with the intention
of injecting it into some fresh chickens. But, as the story goes,
his supply of chickens was limited, and therefore he used the
previously injected chickens. Again to his surprise, the chick-
ens were completely protected from the disease. Pasteur
hypothesized and proved that aging had weakened the viru-
lence of the pathogen and that such an attenuated strain
might be administered to protect against the disease. He
called this attenuated strain a vaccine (from the Latin vacca,
meaning “cow”), in honor of Jenner’s work with cowpox
inoculation.
Pasteur extended these findings to other diseases, demon-
strating that it was possible to attenuate, or weaken, a
pathogen and administer the attenuated strain as a vaccine.
In a now classic experiment at Pouilly-le-Fort in 1881, Pas-
teur first vaccinated one group of sheep with heat-attenuated
anthrax bacillus (Bacillus anthracis); he then challenged the
vaccinated sheep and some unvaccinated sheep with a viru-
lent culture of the bacillus. All the vaccinated sheep lived, and
all the unvaccinated animals died. These experiments
marked the beginnings of the discipline of immunology. In
FIGURE 1-1 Wood engraving of Louis Pasteur watching Joseph
Meister receive the rabies vaccine. [From Harper’s Weekly 29:836;
courtesy of the National Library of Medicine.]
1885, Pasteur administered his first vaccine to a human, a
young boy who had been bitten repeatedly by a rabid dog
(Figure 1-1). The boy, Joseph Meister, was inoculated with a
series of attenuated rabies virus preparations. He lived and
later became a custodian at the Pasteur Institute.
Early Studies Revealed Humoral and Cellular
Components of the Immune System
Although Pasteur proved that vaccination worked, he did not
understand how. The experimental work of Emil von
Behring and Shibasaburo Kitasato in 1890 gave the first in-
sights into the mechanism of immunity, earning von Behring
the Nobel prize in medicine in 1901 (Table 1-1). Von Behring
and Kitasato demonstrated that serum (the liquid, noncellu-
lar component of coagulated blood) from animals previously
immunized to diphtheria could transfer the immune state to
unimmunized animals. In search of the protective agent, var-
ious researchers during the next decade demonstrated that
an active component from immune serum could neutralize
toxins, precipitate toxins, and agglutinate (clump) bacteria.
In each case, the active agent was named for the activity it ex-
hibited: antitoxin, precipitin, and agglutinin, respectively.
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 3 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
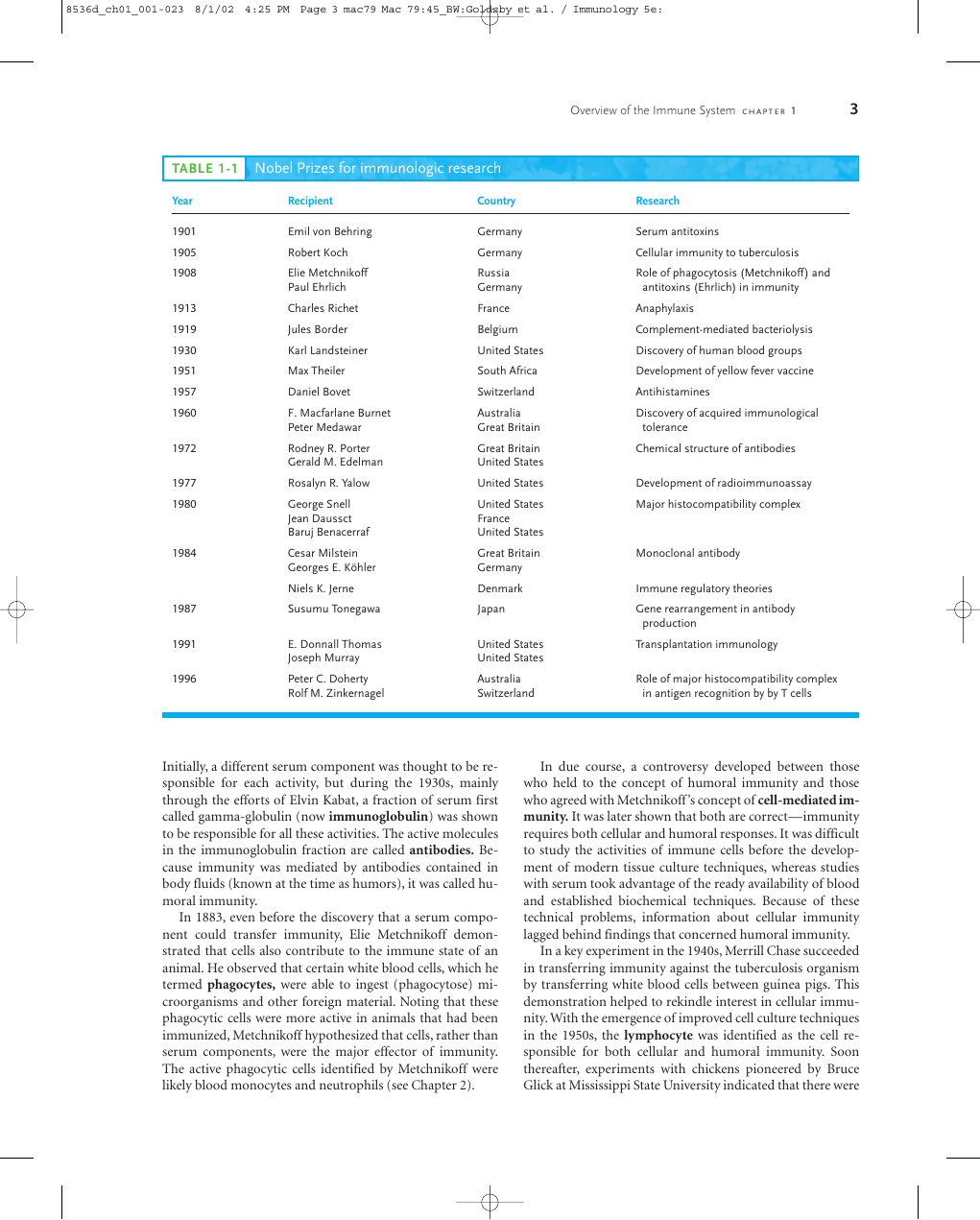
TABLE 1-1 Nobel Prizes for immunologic research
Overview of the Immune System C H A P T E R 1
3
Year
1901
1905
1908
1913
1919
1930
1951
1957
1960
1972
1977
1980
1984
1987
1991
1996
Recipient
Emil von Behring
Robert Koch
Elie Metchnikoff
Paul Ehrlich
Charles Richet
Jules Border
Karl Landsteiner
Max Theiler
Daniel Bovet
F. Macfarlane Burnet
Peter Medawar
Rodney R. Porter
Gerald M. Edelman
Rosalyn R. Yalow
George Snell
Jean Daussct
Baruj Benacerraf
Cesar Milstein
Georges E. Köhler
Niels K. Jerne
Susumu Tonegawa
E. Donnall Thomas
Joseph Murray
Peter C. Doherty
Rolf M. Zinkernagel
Country
Germany
Germany
Russia
Germany
France
Belgium
United States
South Africa
Switzerland
Australia
Great Britain
Great Britain
United States
United States
United States
France
United States
Great Britain
Germany
Denmark
Japan
United States
United States
Australia
Switzerland
Research
Serum antitoxins
Cellular immunity to tuberculosis
Role of phagocytosis (Metchnikoff) and
antitoxins (Ehrlich) in immunity
Anaphylaxis
Complement-mediated bacteriolysis
Discovery of human blood groups
Development of yellow fever vaccine
Antihistamines
Discovery of acquired immunological
tolerance
Chemical structure of antibodies
Development of radioimmunoassay
Major histocompatibility complex
Monoclonal antibody
Immune regulatory theories
Gene rearrangement in antibody
production
Transplantation immunology
Role of major histocompatibility complex
in antigen recognition by by T cells
Initially, a different serum component was thought to be re-
sponsible for each activity, but during the 1930s, mainly
through the efforts of Elvin Kabat, a fraction of serum first
called gamma-globulin (now immunoglobulin) was shown
to be responsible for all these activities. The active molecules
in the immunoglobulin fraction are called antibodies. Be-
cause immunity was mediated by antibodies contained in
body fluids (known at the time as humors), it was called hu-
moral immunity.
In 1883, even before the discovery that a serum compo-
nent could transfer immunity, Elie Metchnikoff demon-
strated that cells also contribute to the immune state of an
animal. He observed that certain white blood cells, which he
termed phagocytes, were able to ingest (phagocytose) mi-
croorganisms and other foreign material. Noting that these
phagocytic cells were more active in animals that had been
immunized, Metchnikoff hypothesized that cells, rather than
serum components, were the major effector of immunity.
The active phagocytic cells identified by Metchnikoff were
likely blood monocytes and neutrophils (see Chapter 2).
In due course, a controversy developed between those
who held to the concept of humoral immunity and those
who agreed with Metchnikoff ’s concept of cell-mediated im-
munity. It was later shown that both are correct—immunity
requires both cellular and humoral responses. It was difficult
to study the activities of immune cells before the develop-
ment of modern tissue culture techniques, whereas studies
with serum took advantage of the ready availability of blood
and established biochemical techniques. Because of these
technical problems, information about cellular immunity
lagged behind findings that concerned humoral immunity.
In a key experiment in the 1940s, Merrill Chase succeeded
in transferring immunity against the tuberculosis organism
by transferring white blood cells between guinea pigs. This
demonstration helped to rekindle interest in cellular immu-
nity. With the emergence of improved cell culture techniques
in the 1950s, the lymphocyte was identified as the cell re-
sponsible for both cellular and humoral immunity. Soon
thereafter, experiments with chickens pioneered by Bruce
Glick at Mississippi State University indicated that there were
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 4 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
4
P A R T I
Introduction
two types of lymphocytes: T lymphocytes derived from the
thymus mediated cellular immunity, and B lymphocytes
from the bursa of Fabricius (an outgrowth of the cloaca in
birds) were involved in humoral immunity. The controversy
about the roles of humoral and cellular immunity was re-
solved when the two systems were shown to be intertwined,
and that both systems were necessary for the immune
response.
Early Theories Attempted to Explain
the Specificity of the Antibody–
Antigen Interaction
One of the greatest enigmas facing early immunologists was
the specificity of the antibody molecule for foreign material,
or antigen (the general term for a substance that binds with
a specific antibody). Around 1900, Jules Bordet at the Pasteur
Institute expanded the concept of immunity by demonstrat-
ing specific immune reactivity to nonpathogenic substances,
such as red blood cells from other species. Serum from an an-
imal inoculated previously with material that did not cause
infection would react with this material in a specific manner,
and this reactivity could be passed to other animals by trans-
ferring serum from the first. The work of Karl Landsteiner
and those who followed him showed that injecting an animal
with almost any organic chemical could induce production
of antibodies that would bind specifically to the chemical.
These studies demonstrated that antibodies have a capacity
for an almost unlimited range of reactivity, including re-
sponses to compounds that had only recently been synthe-
sized in the laboratory and had not previously existed in
nature. In addition, it was shown that molecules differing in
the smallest detail could be distinguished by their reactivity
with different antibodies. Two major theories were proposed
to account for this specificity: the selective theory and the in-
structional theory.
The earliest conception of the selective theory dates to Paul
Ehrlich in 1900. In an attempt to explain the origin of serum
antibody, Ehrlich proposed that cells in the blood expressed a
variety of receptors, which he called “side-chain receptors,”
that could react with infectious agents and inactivate them.
Borrowing a concept used by Emil Fischer in 1894 to explain
the interaction between an enzyme and its substrate, Ehrlich
proposed that binding of the receptor to an infectious agent
was like the fit between a lock and key. Ehrlich suggested that
interaction between an infectious agent and a cell-bound
receptor would induce the cell to produce and release more
receptors with the same specificity. According to Ehrlich’s
theory, the specificity of the receptor was determined before
its exposure to antigen, and the antigen selected the appro-
priate receptor. Ultimately all aspects of Ehrlich’s theory
would be proven correct with the minor exception that the
“receptor” exists as both a soluble antibody molecule and as a
cell-bound receptor; it is the soluble form that is secreted
rather than the bound form released.
In the 1930s and 1940s, the selective theory was chal-
lenged by various instructional theories, in which antigen
played a central role in determining the specificity of the an-
tibody molecule. According to the instructional theories, a
particular antigen would serve as a template around which
antibody would fold. The antibody molecule would thereby
assume a configuration complementary to that of the antigen
template. This concept was first postulated by Friedrich
Breinl and Felix Haurowitz about 1930 and redefined in the
1940s in terms of protein folding by Linus Pauling. The in-
structional theories were formally disproved in the 1960s, by
which time information was emerging about the structure of
DNA, RNA, and protein that would offer new insights into
the vexing problem of how an individual could make anti-
bodies against almost anything.
In the 1950s, selective theories resurfaced as a result of
new experimental data and, through the insights of Niels
Jerne, David Talmadge, and F. Macfarlane Burnet, were re-
fined into a theory that came to be known as the clonal-
selection theory. According to this theory, an individual
lymphocyte expresses membrane receptors that are specific
for a distinct antigen. This unique receptor specificity is de-
termined before the lymphocyte is exposed to the antigen.
Binding of antigen to its specific receptor activates the cell,
causing it to proliferate into a clone of cells that have the
same immunologic specificity as the parent cell. The clonal-
selection theory has been further refined and is now accepted
as the underlying paradigm of modern immunology.
The Immune System Includes Innate and
Adaptive Components
Immunity—the state of protection from infectious disease
—has both a less specific and more specific component. The
less specific component, innate immunity, provides the first
line of defense against infection. Most components of innate
immunity are present before the onset of infection and con-
stitute a set of disease-resistance mechanisms that are not
specific to a particular pathogen but that include cellular and
molecular components that recognize classes of molecules
peculiar to frequently encountered pathogens. Phagocytic
cells, such as macrophages and neutrophils, barriers such as
skin, and a variety of antimicrobial compounds synthesized
by the host all play important roles in innate immunity. In
contrast to the broad reactivity of the innate immune sys-
tem, which is uniform in all members of a species, the spe-
cific component, adaptive immunity, does not come into
play until there is an antigenic challenge to the organism.
Adaptive immunity responds to the challenge with a high de-
gree of specificity as well as the remarkable property of
“memory.” Typically, there is an adaptive immune response
against an antigen within five or six days after the initial ex-
posure to that antigen. Exposure to the same antigen some
time in the future results in a memory response: the immune
response to the second challenge occurs more quickly than
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 5 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
Overview of the Immune System C H A P T E R 1
5
the first, is stronger, and is often more effective in neutraliz-
ing and clearing the pathogen. The major agents of adaptive
immunity are lymphocytes and the antibodies and other
molecules they produce.
Because adaptive immune responses require some time to
marshal, innate immunity provides the first line of defense
during the critical period just after the host’s exposure to a
pathogen. In general, most of the microorganisms encoun-
tered by a healthy individual are readily cleared within a few
days by defense mechanisms of the innate immune system
before they activate the adaptive immune system.
Innate Immunity
Innate immunity can be seen to comprise four types of de-
fensive barriers: anatomic, physiologic, phagocytic, and in-
flammatory (Table 1-2).
The Skin and the Mucosal Surfaces Provide
Protective Barriers Against Infection
Physical and anatomic barriers that tend to prevent the entry
of pathogens are an organism’s first line of defense against in-
fection. The skin and the surface of mucous membranes are
included in this category because they are effective barriers to
the entry of most microorganisms. The skin consists of two
distinct layers: a thinner outer layer—the epidermis—and a
thicker layer—the dermis. The epidermis contains several
layers of tightly packed epithelial cells. The outer epidermal
layer consists of dead cells and is filled with a waterproofing
protein called keratin. The dermis, which is composed of
connective tissue, contains blood vessels, hair follicles, seba-
ceous glands, and sweat glands. The sebaceous glands are as-
sociated with the hair follicles and produce an oily secretion
called sebum. Sebum consists of lactic acid and fatty acids,
which maintain the pH of the skin between 3 and 5; this pH
inhibits the growth of most microorganisms. A few bacteria
that metabolize sebum live as commensals on the skin and
sometimes cause a severe form of acne. One acne drug,
isotretinoin (Accutane), is a vitamin A derivative that pre-
vents the formation of sebum.
Breaks in the skin resulting from scratches, wounds, or
abrasion are obvious routes of infection. The skin may also
be penetrated by biting insects (e.g., mosquitoes, mites, ticks,
fleas, and sandflies); if these harbor pathogenic organisms,
they can introduce the pathogen into the body as they feed.
The protozoan that causes malaria, for example, is deposited
in humans by mosquitoes when they take a blood meal. Sim-
ilarly, bubonic plague is spread by the bite of fleas, and Lyme
disease is spread by the bite of ticks.
The conjunctivae and the alimentary, respiratory, and
urogenital tracts are lined by mucous membranes, not by the
dry, protective skin that covers the exterior of the body. These
TABLE 1-2
Summary of nonspecific host defenses
Type
Mechanism
Anatomic barriers
Skin
Mucous membranes
Physiologic barriers
Temperature
Low pH
Chemical mediators
Mechanical barrier retards entry of microbes.
Acidic environment (pH 3–5) retards growth of microbes.
Normal flora compete with microbes for attachment sites and nutrients.
Mucus entraps foreign microorganisms.
Cilia propel microorganisms out of body.
Normal body temperature inhibits growth of some pathogens.
Fever response inhibits growth of some pathogens.
Acidity of stomach contents kills most ingested microorganisms.
Lysozyme cleaves bacterial cell wall.
Interferon induces antiviral state in uninfected cells.
Complement lyses microorganisms or facilitates phagocytosis.
Toll-like receptors recognize microbial molecules, signal cell to secrete immunostimulatory cytokines.
Collectins disrupt cell wall of pathogen.
Phagocytic/endocytic barriers
Various cells internalize (endocytose) and break down foreign macromolecules.
Specialized cells (blood monocytes, neutrophils, tissue macrophages) internalize
(phagocytose), kill, and digest whole microorganisms.
Inflammatory barriers
Tissue damage and infection induce leakage of vascular fluid, containing serum proteins with
antibacterial activity, and influx of phagocytic cells into the affected area.
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 6 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
6
P A R T I
Introduction
membranes consist of an outer epithelial layer and an under-
lying layer of connective tissue. Although many pathogens
enter the body by binding to and penetrating mucous mem-
branes, a number of nonspecific defense mechanisms tend to
prevent this entry. For example, saliva, tears, and mucous se-
cretions act to wash away potential invaders and also contain
antibacterial or antiviral substances. The viscous fluid called
mucus, which is secreted by epithelial cells of mucous mem-
branes, entraps foreign microorganisms. In the lower respi-
ratory tract, the mucous membrane is covered by cilia,
hairlike protrusions of the epithelial-cell membranes. The
synchronous movement of cilia propels mucus-entrapped
microorganisms from these tracts. In addition, nonpatho-
genic organisms tend to colonize the epithelial cells of mu-
cosal surfaces. These normal flora generally outcompete
pathogens for attachment sites on the epithelial cell surface
and for necessary nutrients.
Some organisms have evolved ways of escaping these de-
fense mechanisms and thus are able to invade the body
through mucous membranes. For example, influenza virus
(the agent that causes flu) has a surface molecule that enables
it to attach firmly to cells in mucous membranes of the respi-
ratory tract, preventing the virus from being swept out by the
ciliated epithelial cells. Similarly, the organism that causes
gonorrhea has surface projections that allow it to bind to ep-
ithelial cells in the mucous membrane of the urogenital tract.
Adherence of bacteria to mucous membranes is due to inter-
actions between hairlike protrusions on a bacterium, called
fimbriae or pili, and certain glycoproteins or glycolipids that
are expressed only by epithelial cells of the mucous mem-
brane of particular tissues (Figure 1-2). For this reason, some
FIGURE 1-2 Electron micrograph of rod-shaped Escherichia coli
bacteria adhering to surface of epithelial cells of the urinary tract.
[From N. Sharon and H. Lis, 1993, Sci. Am. 268(1):85; photograph
courtesy of K. Fujita.]
tissues are susceptible to bacterial invasion, whereas others
are not.
Physiologic Barriers to Infection Include
General Conditions and Specific Molecules
The physiologic barriers that contribute to innate immu-
nity include temperature, pH, and various soluble and cell-
associated molecules. Many species are not susceptible to cer-
tain diseases simply because their normal body temperature
inhibits growth of the pathogens. Chickens, for example,
have innate immunity to anthrax because their high body
temperature inhibits the growth of the bacteria. Gastric acid-
ity is an innate physiologic barrier to infection because very
few ingested microorganisms can survive the low pH of the
stomach contents. One reason newborns are susceptible to
some diseases that do not afflict adults is that their stomach
contents are less acid than those of adults.
A variety of soluble factors contribute to innate immu-
nity, among them the soluble proteins lysozyme, interferon,
and complement. Lysozyme, a hydrolytic enzyme found in
mucous secretions and in tears, is able to cleave the peptido-
glycan layer of the bacterial cell wall. Interferon comprises a
group of proteins produced by virus-infected cells. Among
the many functions of the interferons is the ability to bind to
nearby cells and induce a generalized antiviral state. Comple-
ment, examined in detail in Chapter 13, is a group of serum
proteins that circulate in an inactive state. A variety of spe-
cific and nonspecific immunologic mechanisms can convert
the inactive forms of complement proteins into an active
state with the ability to damage the membranes of patho-
genic organisms, either destroying the pathogens or facilitat-
ing their clearance. Complement may function as an effector
system that is triggered by binding of antibodies to certain
cell surfaces, or it may be activated by reactions between
complement molecules and certain components of microbial
cell walls. Reactions between complement molecules or frag-
ments of complement molecules and cellular receptors trig-
ger activation of cells of the innate or adaptive immune
systems. Recent studies on collectins indicate that these sur-
factant proteins may kill certain bacteria directly by disrupt-
ing their lipid membranes or, alternatively, by aggregating the
bacteria to enhance their susceptibility to phagocytosis.
Many of the molecules involved in innate immunity have
the property of pattern recognition, the ability to recognize a
given class of molecules. Because there are certain types of mol-
ecules that are unique to microbes and never found in multi-
cellular organisms, the ability to immediately recognize and
combat invaders displaying such molecules is a strong feature
of innate immunity. Molecules with pattern recognition ability
may be soluble, like lysozyme and the complement compo-
nents described above, or they may be cell-associated receptors.
Among the class of receptors designated the toll-like receptors
(TLRs), TLR2 recognizes the lipopolysaccharide (LPS) found
on Gram-negative bacteria. It has long been recognized that
�
8536d_ch01_007 9/5/02 11:47 AM Page 7 mac46 mac46:385_reb:
Overview of the Immune System C H A P T E R 1
7
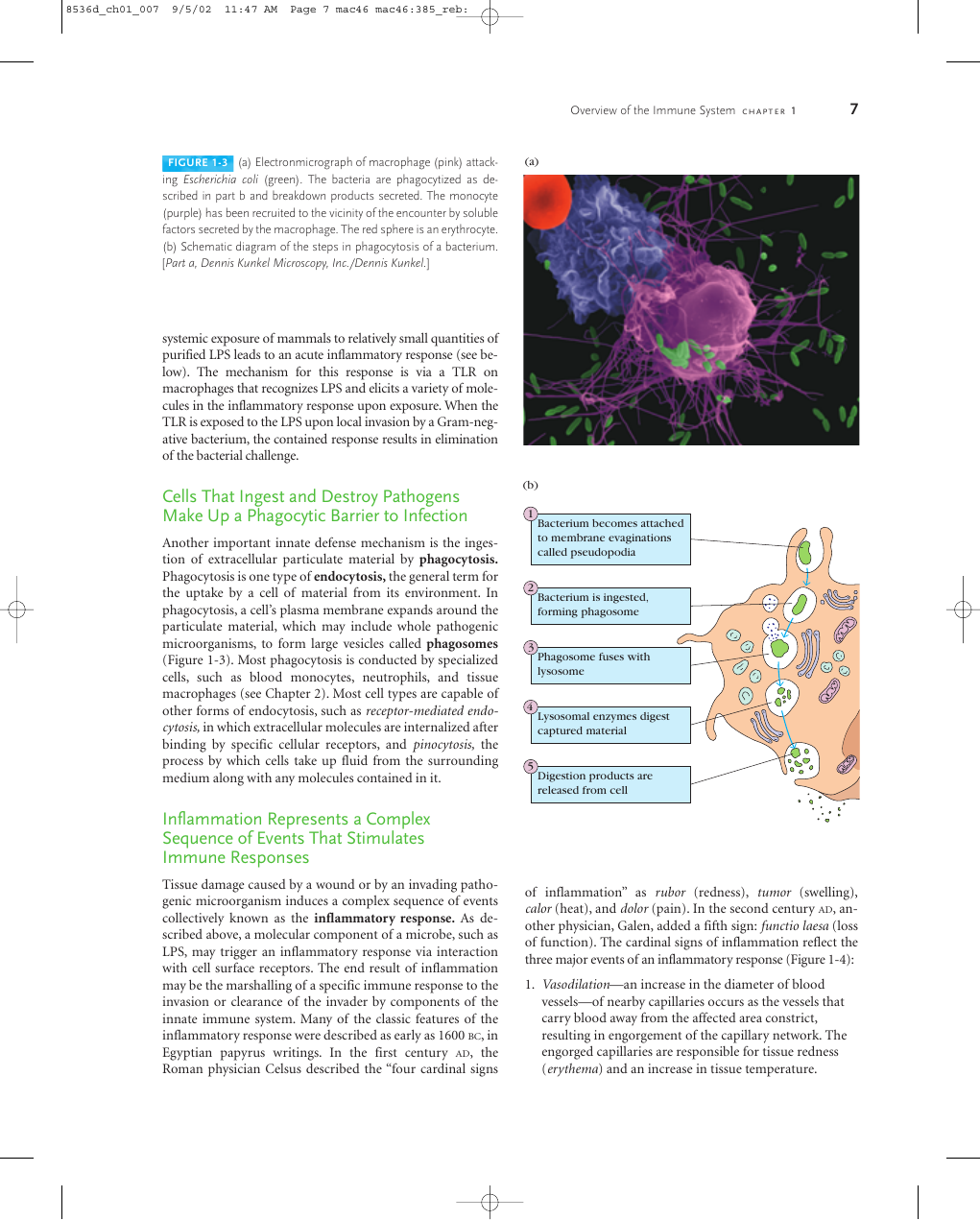
FIGURE 1-3 (a) Electronmicrograph of macrophage (pink) attack-
ing Escherichia coli (green). The bacteria are phagocytized as de-
scribed in part b and breakdown products secreted. The monocyte
(purple) has been recruited to the vicinity of the encounter by soluble
factors secreted by the macrophage. The red sphere is an erythrocyte.
(b) Schematic diagram of the steps in phagocytosis of a bacterium.
[Part a, Dennis Kunkel Microscopy, Inc./Dennis Kunkel.]
(a)
systemic exposure of mammals to relatively small quantities of
purified LPS leads to an acute inflammatory response (see be-
low). The mechanism for this response is via a TLR on
macrophages that recognizes LPS and elicits a variety of mole-
cules in the inflammatory response upon exposure. When the
TLR is exposed to the LPS upon local invasion by a Gram-neg-
ative bacterium, the contained response results in elimination
of the bacterial challenge.
Cells That Ingest and Destroy Pathogens
Make Up a Phagocytic Barrier to Infection
Another important innate defense mechanism is the inges-
tion of extracellular particulate material by phagocytosis.
Phagocytosis is one type of endocytosis, the general term for
the uptake by a cell of material from its environment. In
phagocytosis, a cell’s plasma membrane expands around the
particulate material, which may include whole pathogenic
microorganisms, to form large vesicles called phagosomes
(Figure 1-3). Most phagocytosis is conducted by specialized
cells, such as blood monocytes, neutrophils, and tissue
macrophages (see Chapter 2). Most cell types are capable of
other forms of endocytosis, such as receptor-mediated endo-
cytosis, in which extracellular molecules are internalized after
binding by specific cellular receptors, and pinocytosis, the
process by which cells take up fluid from the surrounding
medium along with any molecules contained in it.
Inflammation Represents a Complex
Sequence of Events That Stimulates
Immune Responses
Tissue damage caused by a wound or by an invading patho-
genic microorganism induces a complex sequence of events
collectively known as the inflammatory response. As de-
scribed above, a molecular component of a microbe, such as
LPS, may trigger an inflammatory response via interaction
with cell surface receptors. The end result of inflammation
may be the marshalling of a specific immune response to the
invasion or clearance of the invader by components of the
innate immune system. Many of the classic features of the
inflammatory response were described as early as 1600 BC, in
Egyptian papyrus writings. In the first century AD, the
Roman physician Celsus described the “four cardinal signs
(b)
1
2
3
4
5
Bacterium becomes attached
to membrane evaginations
called pseudopodia
Bacterium is ingested,
forming phagosome
Phagosome fuses with
lysosome
Lysosomal enzymes digest
captured material
Digestion products are
released from cell
of
inflammation” as rubor (redness), tumor (swelling),
calor (heat), and dolor (pain). In the second century AD, an-
other physician, Galen, added a fifth sign: functio laesa (loss
of function). The cardinal signs of inflammation reflect the
three major events of an inflammatory response (Figure 1-4):
1. Vasodilation—an increase in the diameter of blood
vessels—of nearby capillaries occurs as the vessels that
carry blood away from the affected area constrict,
resulting in engorgement of the capillary network. The
engorged capillaries are responsible for tissue redness
(erythema) and an increase in tissue temperature.
�
8536d_ch01_001-023 8/1/02 4:25 PM Page 8 mac79 Mac 79:45_BW:Goldsby et al. / Immunology 5e:
8
P A R T I
Introduction
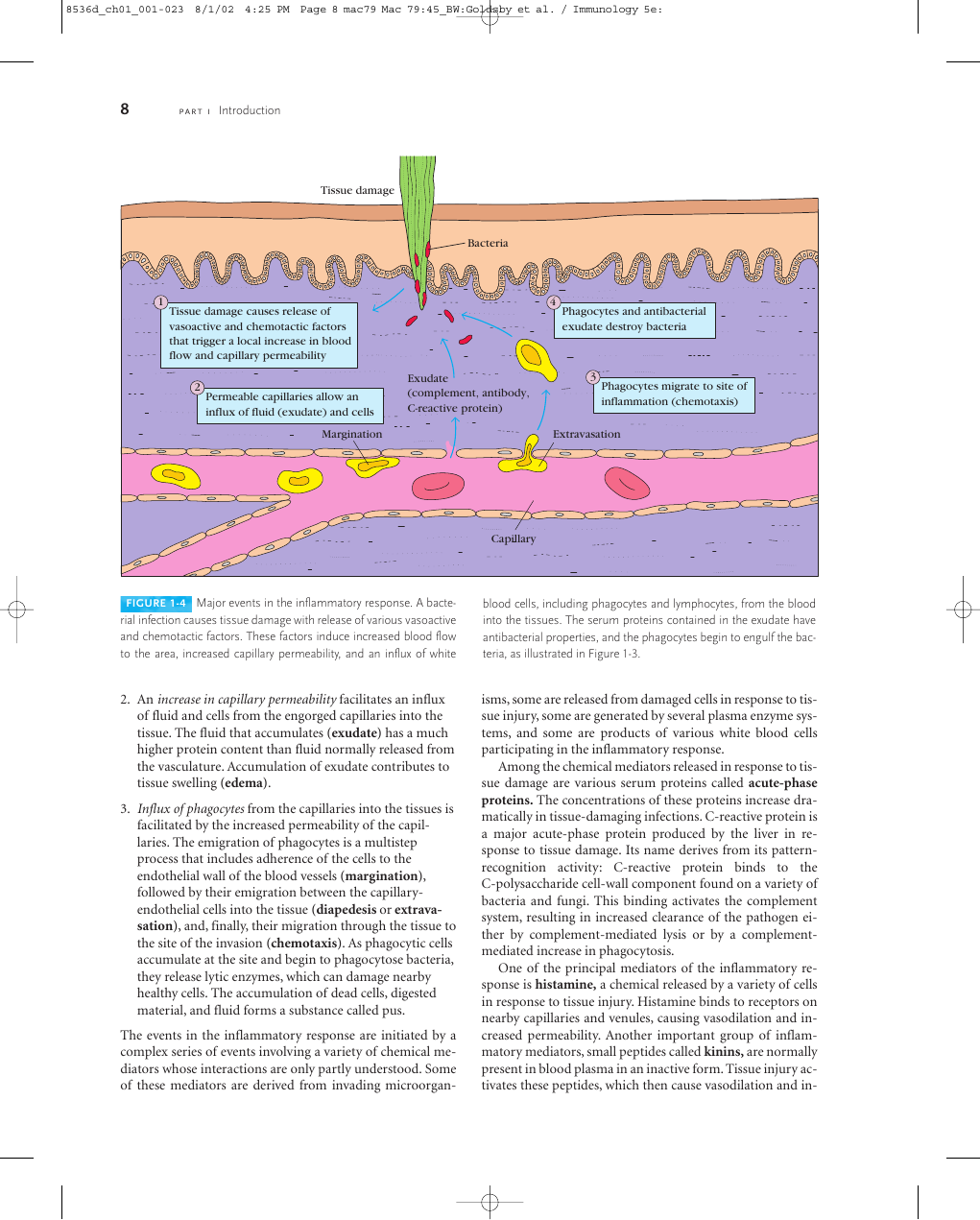
Tissue damage
Bacteria
1
Tissue damage causes release of
vasoactive and chemotactic factors
that trigger a local increase in blood
flow and capillary permeability
4
Phagocytes and antibacterial
exudate destroy bacteria
2
Permeable capillaries allow an
influx of fluid (exudate) and cells
Exudate
(complement, antibody,
C-reactive protein)
3
Phagocytes migrate to site of
inflammation (chemotaxis)
Margination
Extravasation
Capillary
FIGURE 1-4 Major events in the inflammatory response. A bacte-
rial infection causes tissue damage with release of various vasoactive
and chemotactic factors. These factors induce increased blood flow
to the area, increased capillary permeability, and an influx of white
blood cells, including phagocytes and lymphocytes, from the blood
into the tissues. The serum proteins contained in the exudate have
antibacterial properties, and the phagocytes begin to engulf the bac-
teria, as illustrated in Figure 1-3.
2. An increase in capillary permeability facilitates an influx
of fluid and cells from the engorged capillaries into the
tissue. The fluid that accumulates (exudate) has a much
higher protein content than fluid normally released from
the vasculature. Accumulation of exudate contributes to
tissue swelling (edema).
3. Influx of phagocytes from the capillaries into the tissues is
facilitated by the increased permeability of the capil-
laries. The emigration of phagocytes is a multistep
process that includes adherence of the cells to the
endothelial wall of the blood vessels (margination),
followed by their emigration between the capillary-
endothelial cells into the tissue (diapedesis or extrava-
sation), and, finally, their migration through the tissue to
the site of the invasion (chemotaxis). As phagocytic cells
accumulate at the site and begin to phagocytose bacteria,
they release lytic enzymes, which can damage nearby
healthy cells. The accumulation of dead cells, digested
material, and fluid forms a substance called pus.
The events in the inflammatory response are initiated by a
complex series of events involving a variety of chemical me-
diators whose interactions are only partly understood. Some
of these mediators are derived from invading microorgan-
isms, some are released from damaged cells in response to tis-
sue injury, some are generated by several plasma enzyme sys-
tems, and some are products of various white blood cells
participating in the inflammatory response.
Among the chemical mediators released in response to tis-
sue damage are various serum proteins called acute-phase
proteins. The concentrations of these proteins increase dra-
matically in tissue-damaging infections. C-reactive protein is
a major acute-phase protein produced by the liver in re-
sponse to tissue damage. Its name derives from its pattern-
recognition activity: C-reactive protein binds to the
C-polysaccharide cell-wall component found on a variety of
bacteria and fungi. This binding activates the complement
system, resulting in increased clearance of the pathogen ei-
ther by complement-mediated lysis or by a complement-
mediated increase in phagocytosis.
One of the principal mediators of the inflammatory re-
sponse is histamine, a chemical released by a variety of cells
in response to tissue injury. Histamine binds to receptors on
nearby capillaries and venules, causing vasodilation and in-
creased permeability. Another important group of inflam-
matory mediators, small peptides called kinins, are normally
present in blood plasma in an inactive form. Tissue injury ac-
tivates these peptides, which then cause vasodilation and in-
�













 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc