A Novel Channel Model for Molecular Communications
Based on Inter-Cellular Calcium Wave
Hengtai Chang1, Ji Bian1, Jian Sun1,2, Wensheng Zhang1, and Cheng-Xiang Wang3,∗
1Shandong Provincial Key Lab of Wireless Communication Technologies,
Shandong Unviersity, Jinan, Shandong, 250100, P.R.China
2State Key Lab. of Millimeter Waves, Southeast University, Nanjing, 210096, P.R.China
3Institute of Sensors, Signals and Systems, School of Engineering & Physical Sciences,
Heriot-Watt University, Edinburgh, EH14 4AS, UK
hunter_chang@126.com,bianjimail@163.com,{sunjian,zhangwsh}@sdu.edu.cn,
cheng-xiang.wang@hw.ac.uk
Abstract. Calcium signalling is a good bio-inspired method for molecular communication
due to the advantages of biocompatibility, stability, and long communication range. In this
paper, we investigate a few channel characteristics of calcium signaling transfer systems
including propagation distance and time delay based on a novel inter-cellular calcium wave
(ICW) propagation model. Our model is the first one that can investigate the impact of
some exclusive parameters in ICW (e.g., the gap junction permeability). Understanding
the channel transfer characteristics of ICW can provide a significant reference for the
calcium signaling application in molecular communication. In the future, theoretical and
simulation results in this paper can help in the design of molecular communication systems
between nanodevices.
Key words: Molecular communication, inter-cellular calcium wave, channel characteristics.
1 Introduction
In recent years, the rapid development of nanotechnologies provides many new applications
in biomedical, industrial, and military fields [1]. In nanometer scale, the most basic unit is
called nano-machine [2] and each nano-machine can only perform simple tasks like sensing,
computing, and drug delivery. Therefore, cooperation of different nano-machines is significant
for nano-machines to do complex tasks. Nano-sized communication allowing nano-machines to
pass instructions and sharing informations is an important part of cooperation of nano-machines.
Molecular communication is a type of promising nano-sized communication technology and
can be a good compensation to traditional communication mode due to advantages of biocom-
patibility, small scale, and high energy efficiency [3]. In the molecular communication system,
transmitted information is encoded in molecules and communicated by diffusion or molecular mo-
tor. By means of molecular communications, nano-networks between nano-machines can enable
nano-machines to exchange messages and work cooperatively [4].
This paper concentrates on the bio-inspired molecular communication approach, which mean-
s that develop molecular communication from communication mechanism existing in biological
structures. In nature, cells in organism transfer significant information to other cells using infor-
mation objects. Bio-inspired approach utilizes communication mechanism existing in organism
such as intercellular Ca2+, Na+ wave propagation, and hormone traveling, to develop new ad-
vanced molecular communication technology. For this approach, bio-inspired molecular commu-
nication can work on both bio-organism and nano-machines that offer compatible solution for
in-body communication scenario.
�
2
H. Chang et al.
Calcium signal is a type of bio-inspired molecular communication method based on ICW [5].
Many studies suggested that this kind of communication method exists widely in nature [6] [7]. As
shown in Fig. 1, human smooth muscle coupling in intestines and stomach is mediated by Ca2+
release. In human astrocytes, the calcium wave plays an important role in information transfer
in remote parts of the brain. Calcium signal is also a common phenomenon in epithelial cells,
the calcium oscillation resulting from a simple regenerative is of vital importance for system
equilibrium. In Ca2+ signaling, connected cells array can serve as a communication channel
connecting the transmitter and receiver. The inter-cellular calcium wave can be transferred to
the touching neighbor cells through the gap junctions, which results in the intercellular Ca2+
wave propagation.
Calcium signal have been studied by many researchers for a long time. In [10], a relay channel
model based on ICW was proposed and communication capacity of a Ca2+ relay channel was
computed. In [11], a linear channel model for intra/inter-cellular Ca2+ molecular communication
based on Ca2+ signal was investigated and some channel characteristics were derived. However,
these Ca2+ channel models mainly considered the effect of Ca2+ diffusion and Ca2+ induced
Ca2+ release (CICR). Inositol 1, 4, 5-triphosphate (IP3) induced Ca2+ release was rarely taken
into account in these channel models. According to the latest study [5], in some certain type of
cells such as epithelial cells, ICW is mainly caused by transmission of IP3 between adjacent cells
instead of calcium itself. This paper investigates the molecular communication channel model
based on IP3 induced ICW. Close-form solutions for channel characteristics and channel capacity
based on information theory are obtained, which will play important roles in communication
system design and performance evaluation.
Fig. 1. Locations of cells having inter-cellular Ca2+ wave propagation.
The rest of this paper can be divided in four parts. In Section 2, the mathematical model
of cytosolic Ca2+ concentration oscillation and gap junction relevant to channel modeling is
described and analyzed. In Section 3, some significant channel characteristics are analyzed based
on a mathematical model. Binary channel capacity is computed in Section 4. Section 5 concludes
the whole paper and points out the future works.
�
A Novel Channel Model for Molecular Communications Based on Inter-Cellular Calcium Wave
3
2 Channel Models Based on ICW
2.1 Ca2+ Oscillation Model
Cytosolic Ca2+ serves as a kind of crucial second messenger in inter/inner cellular communica-
tions. The principle of this communication method has been studied by many researchers and
different dynamic models have been proposed to explain the mechanism of ICW [8] [9]. However,
most communication channel models based on ICW mainly considered CICR to describe ICW
communication mechanism, which dose not conform to the reality very well. In this paper, we
refer to the ICW model proposed in [14], which takes IP3 as the main factor triggering the ICW,
and apply this dynamic model to a molecular communication scenario, i.e. lots of cells connected
with each other via gap junction. Then, we simulate the molecular communication process to get
the ICW propagation characteristics based on the Ca2+ channel model.
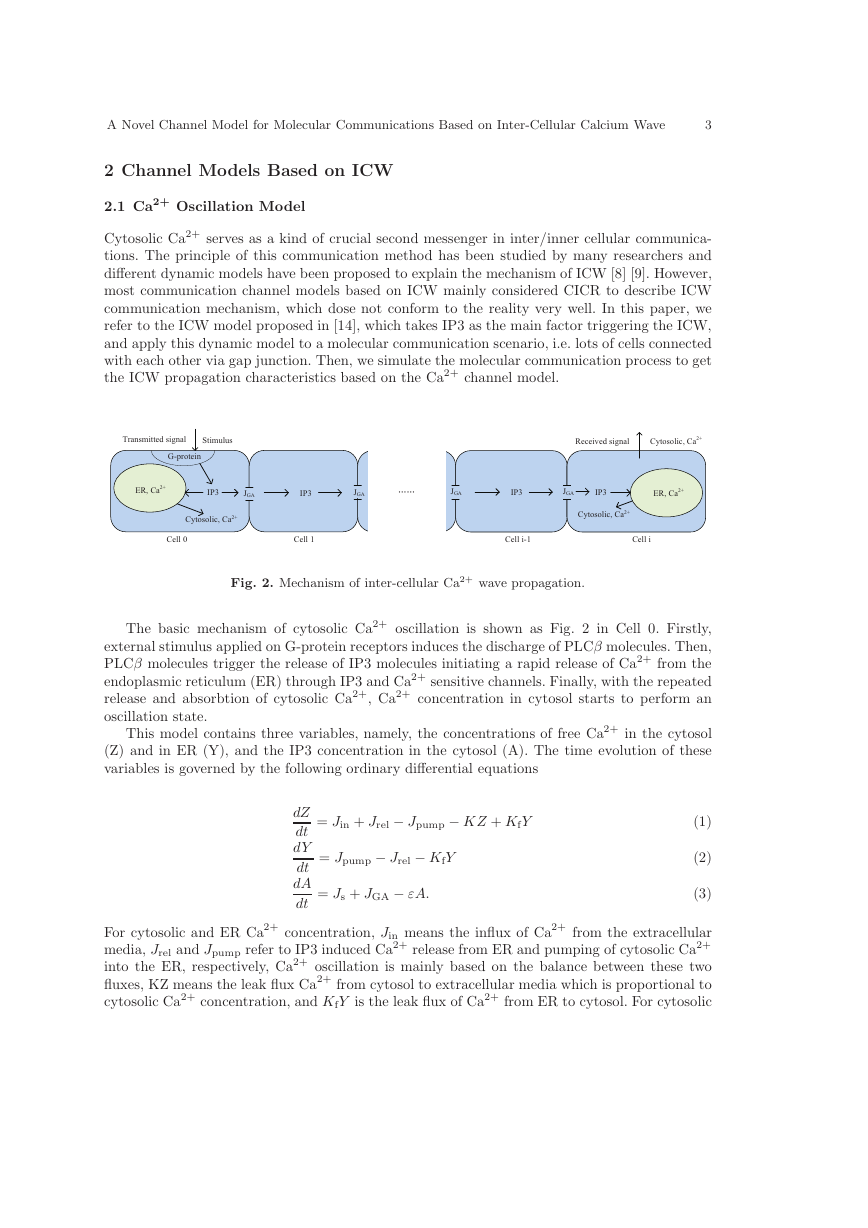
Fig. 2. Mechanism of inter-cellular Ca2+ wave propagation.
The basic mechanism of cytosolic Ca2+ oscillation is shown as Fig. 2 in Cell 0. Firstly,
external stimulus applied on G-protein receptors induces the discharge of PLCβ molecules. Then,
PLCβ molecules trigger the release of IP3 molecules initiating a rapid release of Ca2+ from the
endoplasmic reticulum (ER) through IP3 and Ca2+ sensitive channels. Finally, with the repeated
release and absorbtion of cytosolic Ca2+, Ca2+ concentration in cytosol starts to perform an
oscillation state.
This model contains three variables, namely, the concentrations of free Ca2+ in the cytosol
(Z) and in ER (Y), and the IP3 concentration in the cytosol (A). The time evolution of these
variables is governed by the following ordinary differential equations
dZ
dt
dY
dt
dA
dt
= Jin + Jrel − Jpump − KZ + KfY
= Jpump − Jrel − KfY
= Js + JGA − εA.
(1)
(2)
(3)
For cytosolic and ER Ca2+ concentration, Jin means the influx of Ca2+ from the extracellular
media, Jrel and Jpump refer to IP3 induced Ca2+ release from ER and pumping of cytosolic Ca2+
into the ER, respectively, Ca2+ oscillation is mainly based on the balance between these two
fluxes, KZ means the leak flux Ca2+ from cytosol to extracellular media which is proportional to
cytosolic Ca2+ concentration, and KfY is the leak flux of Ca2+ from ER to cytosol. For cytosolic
�
4
H. Chang et al.
IP3 concentration, Js refers to stimulus induced IP3 release, ε refer to IP3 degration coefficient,
and JGA is the gap junction IP3 flux that will be discussed in next subsection. The function
expressions of the participating fluxes are shown as follows:
Js = βV4
Jin = V0 + V1β
Jpump = VM2
Jrel = VM3
Z 2
K 2
2 + Z 2
Z m
2 + Z m
K m
Y 2
Y + Y 2
K 2
(4)
(5)
(6)
(7)
A4
A + A4 .
K 4
In these equations, V0 refers to a constant input of Ca2+ from extracellular space and V1 is the
maximum rate of stimulus-induced influx of Ca2+ from the extracellular medium. Parameter
β reflects the degree of stimulus that only varies between 0 and 1, V4 is the maximum rate
of stimulus-induced synthesis of IP3. VM2 and VM3 denote the maximum values of Jpump and
Jrel, respectively. Parameters K2, KY, and KA are threshold constants for pumping, release, and
activation of Ca2+ release by Ca2+ and by IP3, respectively. These parameter values are shown
in Table 1.
Table 1. Simulation parameters.
Parameter
Coefficient of leak flux, Kf
Transport coefficient of cytosolic Ca2+, k
Threshold constant for Jpump, K2
Threshold constant for Jrel correlated to A, KA
Threshold constant for Jrel correlated to Y, KY
Threshold constant for Jrel correlated to Z, KZ
Value
10 s−1
0.1 µM
0.2 µM
0.1 µM
0.2 µM
0.5 µM
2 µM s−1
Maximum rate of stimulus-induced influx of Ca2+, V1 2 µM s−1
6 µM s−1
60 µM s−1
2 µM s−1
0.3 s−1
Maximum value of Jrel, VM3
Maximum value of Jin, V4
Ca2+ from the extracellular medium, V0
Maximum value of Jpump, VM2
Coefficient of IP3 degration, ε
Hill coefficient, m
2
2.2 Gap Junction Model
In the nature, one of the important cell-to-cell communication methods is the gap junction com-
munication. In this way, the communication between different cells is achieved by the exchanges
of message molecules like ions, protein, and organelles at the coupling channels of adjacent cells.
This communication mechanism is a meaningful part of ICW propagation.
In our model, IP3 is transmitted by a transmitter cell, and then propagates through the
gap junction crossing a few cells by means of diffusion. During this period, IP3 induces Ca2+
oscillation in passing cells and suffers decay in propagation process. Finally IP3 is received by the
receiver cell and excites the Ca2+ oscillation. The IP3 flux between gap junction coupling cells
�
A Novel Channel Model for Molecular Communications Based on Inter-Cellular Calcium Wave
5
is proportional to the IP3 concentration gradient and gap junction permeability [6]. Therefore,
the IP3 gap junction transmitting mechanism is determined as
JGA = PIP3(Z + − Z) + PIP3(Z − − Z)
(8)
where Z + and Z − are Ca2+ concentration in two different adjacent cells, PIP3 is the IP3 gap
junction permeability and PIP3 is usually unaffected by the IP3 concentration. So, we consider
that PIP3 is independent of IP3 concentration.
3 Results and Analysis
In this section, we study the ICW channel characteristics like maximum propagation distance,
propagation time delay, and calcium oscillation frequency as the function of gap junction per-
meability PIP3 and stimulus intensity β using numerical and simulation methods.
3.1 Calcium Oscillation Condition
Refering to the model expression in Section 2. 1, Ca2+ oscillation is mainly based on the balance
between Jpump and Jrel. IP3 concentration increasing causes the increasing of Jrel and breaks the
steady state of cytosolic Ca2+. Then CICR causes the positive feedback of Jrel and finally gives
rise to Ca2+ oscillation. The condition of Ca2+ oscillation can be expressed as Jpump > Jrel, and
substituting (6)(7) into this condition we can get
VM2
Z m
2 + Z m
Z 2
2 + Z 2 > VM3
K 2
A4
A + A4 > max VM2(KZ
VM3(K2
K m
K 4
Y 2
Y + Y 2
K 2
2 + Z 2)
2 + Z 2) .
A4
A + A4
K 4
Through proper simplification, the condition can be written as
4 = K ′
A
1
)
A > (
EK 4
A
1 − E
E = VM2KZ
2/VM3K2
VM2/VM3,
2, KZ > K2
KZ ≤ K2
(9)
(10)
(11)
(12)
which means that the sum of cytosolic Ca2+ fluxes forms the positive feedback and E is the
critical condition of oscillation state and steady state for variable
A4
A+A4 .
K 4
We take IP3 induced Ca2+ release threshold as K ′
A. In order to prove that a certain IP3
concentration K ′
A excites the calcium concentration oscillation, numerical method is used to
simulate IP3 induced calcium oscillation. We use the system parameters from [14] and assume
that all the cells in the system have the same biological parameters. Utilizing Runge-Kutta
method, we get three variables time evolution of IP3 induced Ca2+ oscillation of different cells.
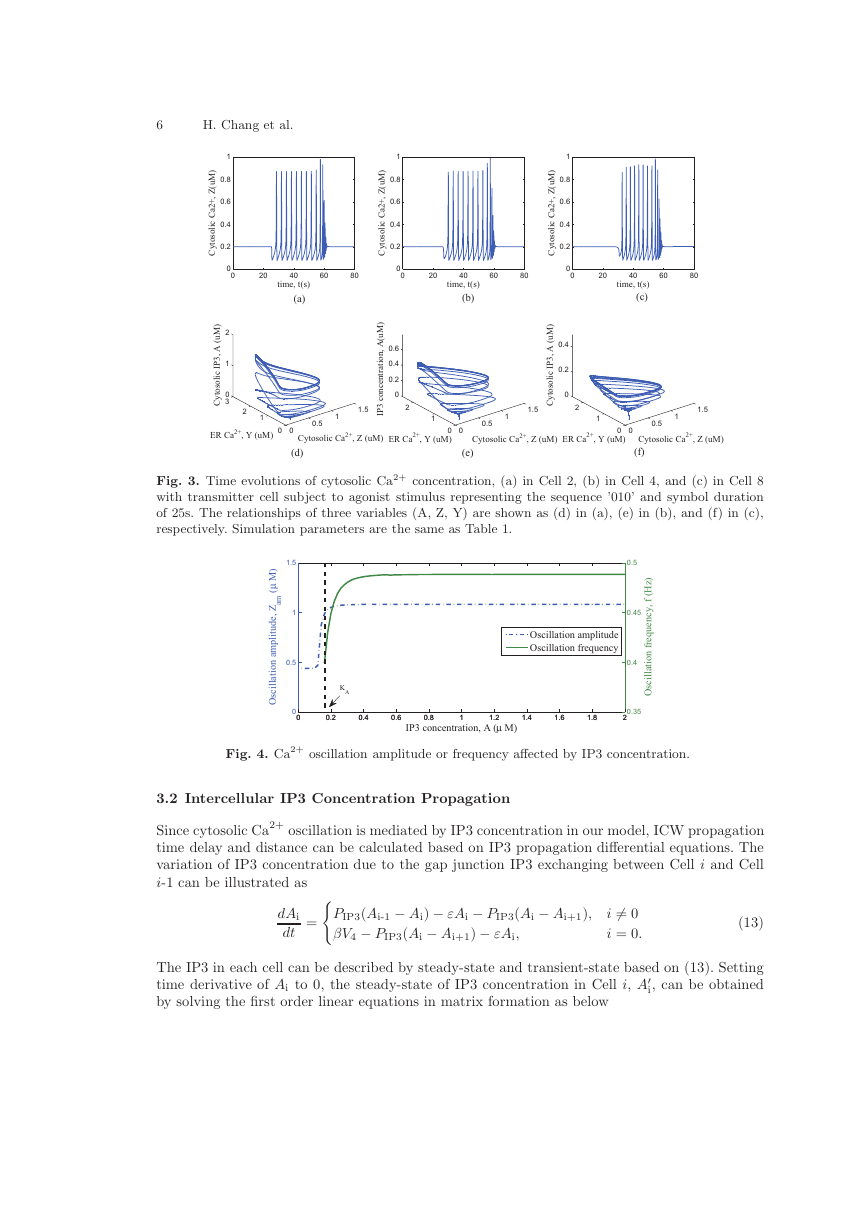
Fig. 3 indicates that the existence of Ca2+ oscillation is controlled by IP3 concentration. The same
IP3 concentration threshold enable the oscillation in different cells. The relationship between IP3
concentration and Ca2+ oscillation amplitude or frequency is shown in Fig. 4. From this numerical
simulation, Ca2+ oscillation amplitude ZAM and frequency fo are related with IP3 concentration
and once IP3 concentration surpasses the threshold value K ′
A, ZAM and fo tend to be a constant.
�
6
H. Chang et al.
Fig. 3. Time evolutions of cytosolic Ca2+ concentration, (a) in Cell 2, (b) in Cell 4, and (c) in Cell 8
with transmitter cell subject to agonist stimulus representing the sequence ’010’ and symbol duration
of 25s. The relationships of three variables (A, Z, Y) are shown as (d) in (a), (e) in (b), and (f) in (c),
respectively. Simulation parameters are the same as Table 1.
µ
µ
Fig. 4. Ca2+ oscillation amplitude or frequency affected by IP3 concentration.
3.2 Intercellular IP3 Concentration Propagation
Since cytosolic Ca2+ oscillation is mediated by IP3 concentration in our model, ICW propagation
time delay and distance can be calculated based on IP3 propagation differential equations. The
variation of IP3 concentration due to the gap junction IP3 exchanging between Cell i and Cell
i-1 can be illustrated as
dAi
dt
=(PIP3(Ai-1 − Ai) − εAi − PIP3(Ai − Ai+1),
βV4 − PIP3(Ai − Ai+1) − εAi,
i 6= 0
i = 0.
(13)
The IP3 in each cell can be described by steady-state and transient-state based on (13). Setting
time derivative of Ai to 0, the steady-state of IP3 concentration in Cell i, A′
i, can be obtained
by solving the first order linear equations in matrix formation as below
�
A Novel Channel Model for Molecular Communications Based on Inter-Cellular Calcium Wave
7
where A is an infinite matrix with the formation
Ax = b
(14)
A =
(PIP3+ε) −PIP3
0
−PIP3
(2PIP3+ε) −PIP3
···
0
···
0
−PIP3
(2PIP3+ε) −PIP3 0 ···
.
...
Here, x and b are infinite vectors, i.e. x =A′
. By solving these
equations, it can be found that steady-state IP3 concentration attenuation ain between Cell i
and Cell i+1 is a function of PIP3 and ε, and can be expressed as
, b =βV4 0 0 · · ·T
2 · · ·T
...
...
0 A′
1 A′
...
ain = 1 −
√ε2 + 4εPIP3 − ε
2PIP3
Therefore, steady-state of IP3 in each cell can be obtained as
A′
i = ainA′
i−1 = ai
inA′
0
and A′
0 is written as
A′
0 =
βV4(√ε2 + 4εPIP3 − ε)
2εPIP3
.
.
(15)
(16)
(17)
Once the IP3 concentration steady-states in each cell is determined, IP3 induced ICW propaga-
tion distance N can be determined as
N logain = log
KA
A′
0
.
(18)
Because of the time consumption of IP3 diffusion in the cytosol, we assume that inter cellular
IP3 propagation starting from cytosolic IP3 concentration is equal to steady state. Then, the
overall response of IP3 concentration in each cell can be written as
0,
PIP3A′
(t − (i − 1)τin))/λ,
i−1(1 − exp(−λ
ainA′
i−1,
t ≤ (i − 1)τin
(i − 1)τin < t ≤ iτin
t > iτin
(19)
Ai(t) =
where λ = PIP3 + ε is the time coefficient and τin is the IP3 propagation time delay for each cell
that can be written as
τin =
1
λ
PIP3 − ainλ
ln(
PIP3
).
(20)
Then, the time delay τi for ICW propagation of Cell i can be calculated by solving Ai(τi) =
KA and the solution is
τi = (i − 1)τin −
1
λ
ln(1 −
KA
A′
i
),
i ≤ N.
(21)
ICW propagation distance and delay can be calculated base on (18) and (21), respectively.
�
8
H. Chang et al.
3.3 Effect of Gap Junction Permeability and Stimulus Intensity
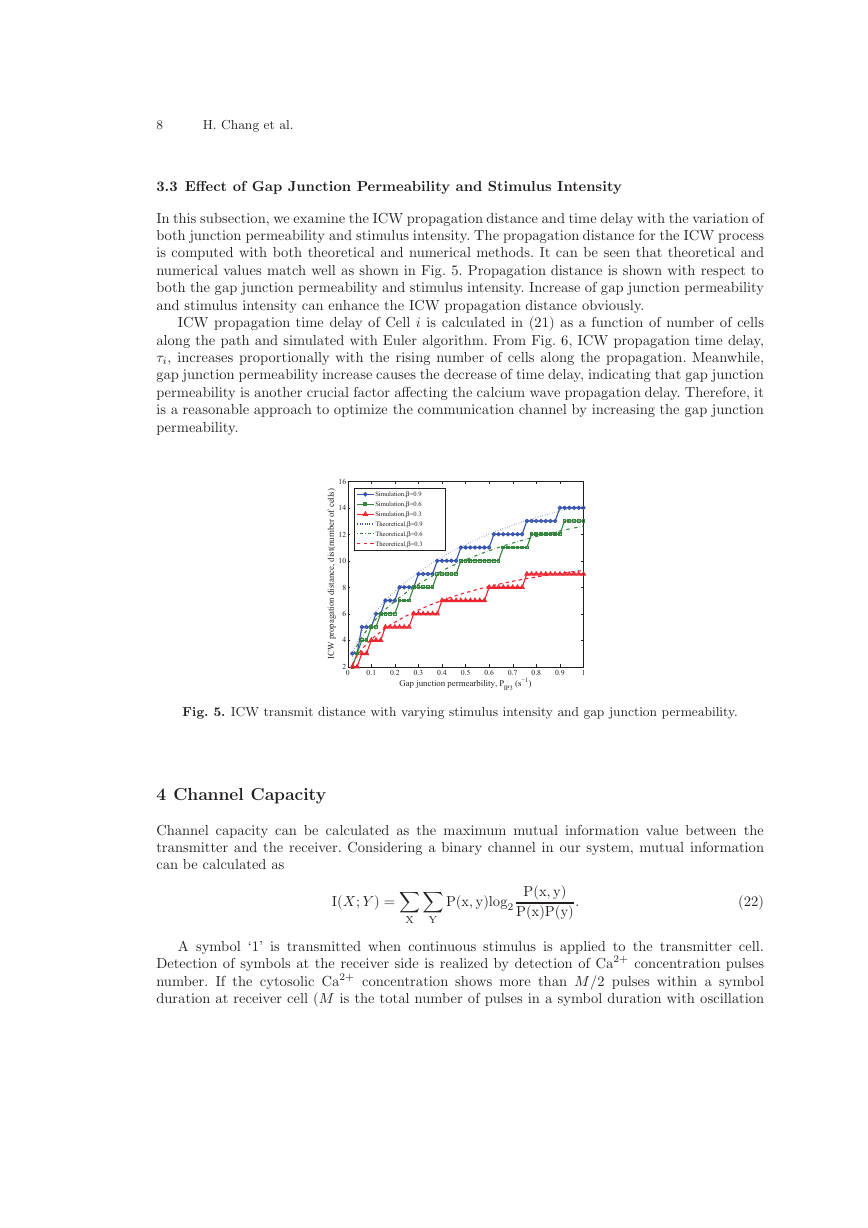
In this subsection, we examine the ICW propagation distance and time delay with the variation of
both junction permeability and stimulus intensity. The propagation distance for the ICW process
is computed with both theoretical and numerical methods. It can be seen that theoretical and
numerical values match well as shown in Fig. 5. Propagation distance is shown with respect to
both the gap junction permeability and stimulus intensity. Increase of gap junction permeability
and stimulus intensity can enhance the ICW propagation distance obviously.
ICW propagation time delay of Cell i is calculated in (21) as a function of number of cells
along the path and simulated with Euler algorithm. From Fig. 6, ICW propagation time delay,
τi, increases proportionally with the rising number of cells along the propagation. Meanwhile,
gap junction permeability increase causes the decrease of time delay, indicating that gap junction
permeability is another crucial factor affecting the calcium wave propagation delay. Therefore, it
is a reasonable approach to optimize the communication channel by increasing the gap junction
permeability.
)
s
l
l
e
c
f
o
r
e
b
m
u
n
(
t
s
i
d
,
e
c
n
a
t
s
i
d
n
o
i
t
a
g
a
p
o
r
p
W
C
I
16
14
12
10
8
6
4
2
0
Simulation,β=0.9
Simulation,β=0.6
Simulation,β=0.3
Theoretical,β=0.9
Theoretical,β=0.6
Theoretical,β=0.3
0.1
0.2
0.3
0.4
0.5
0.6
0.7
(s−1)
IP3
Gap junction permearbility, P
0.8
0.9
1
Fig. 5. ICW transmit distance with varying stimulus intensity and gap junction permeability.
4 Channel Capacity
Channel capacity can be calculated as the maximum mutual information value between the
transmitter and the receiver. Considering a binary channel in our system, mutual information
can be calculated as
I(X; Y ) =XX XY
P(x, y)log2
P(x, y)
P(x)P(y)
.
(22)
A symbol ‘1’ is transmitted when continuous stimulus is applied to the transmitter cell.
Detection of symbols at the receiver side is realized by detection of Ca2+ concentration pulses
number. If the cytosolic Ca2+ concentration shows more than M /2 pulses within a symbol
duration at receiver cell (M is the total number of pulses in a symbol duration with oscillation
�













 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc