Research Article
www.acsami.org
†
Novel Approach of Using Near-Infrared Responsive PEGylated Gold
Nanorod Coated Poly(L‑lactide) Microneedles to Enhance the
Antitumor Efficiency of Docetaxel-Loaded MPEG-PDLLA Micelles for
Treating an A431 Tumor
Ying Hao,
and ZhiYong Qian*,†
†
State Key Laboratory of Biotherapy/Collaborative Innovation Center for Biotherapy, West China Hospital, West China Medical
School, Sichuan University, Chengdu 610041, PR China
‡
Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education, Jianghan University, Wuhan 430056,
PR China
*S Supporting Information
†
YanPeng Jia,
MingLing Dong,
TaoYe Zhang,
JinRong Peng,
YiPing Cao,
‡
†
‡
†
ABSTRACT: The combination of chemotherapy and photothermal therapy (PTT) plays a significant role in synergistic tumor
therapy. However, a high dosage of chemotherapy drugs or photothermal agents may cause series side effects. To overcome these
challenges, we designed a near-infrared (NIR) responsive PEGylated gold nanorod (GNR-PEG) coated poly(L-lactide)
microneedle (PLLA MN) system (GNR-PEG@MN) to enhance antitumor efficiency of docetaxel-loaded MPEG-PDLLA
(MPEG-PDLLA-DTX) micelles for treating an A431 tumor. The as-made GNR-PEG@MNs contained only 31.83 ± 1.22 μg of
GNR-PEG per patch and exhibited excellent heating efficacy both in vitro and in vivo. Meanwhile, GNR-PEG@MN with the
height of 480 μm had good skin insertion ability and was harmless to the skin. On the other hand, GNR-PEG@MN had good
heating transfer ability in vivo, and the tumor sites could reach 50 °C within 5 min. In comparison with chemotherapy and PTT
alone, the combination of low dosage MPEG-PDLLA-DTX micelles (5 mg/kg) and GNR-PEG@MNs completely eradicated the
A431 tumor without recurrence in vivo, demonstrating a remarkable synergetic effect. Hence, GNR-PEG@MN could be a
promising carrier to enhance the antitumor effect of MPEG-PDLLA-DTX micelles for treating superficial tumors and is expected
to have a great potential in clinical translation for human epidermoid cancer therapy.
KEYWORDS: photothermal therapy (PTT), GNR-PEG@MNs, MPEG-PDLLA-DTX micelles, A431 tumor, synergetic effect
■ INTRODUCTION
In recent decades, cancer has been the most common life-
threatening illness to people’s health and has had an upward
trend in its morbidity and mortality.1 Among them, human
epidermoid cancer is a public health problem which has a
growing trend especially in Caucasians.2 Traditional therapies,
such as photodynamic therapy,3 may cause skin DNA damage
and other skin cancer.4,5 Great effort is needed to be devoted to
improve the therapy effect and reduce side effects. Docetaxel
(DTX) is a broad-spectrum antitumor drug which has good
antitumor effect.6,7 It
the half-inhibitory
is reported that
concentration (IC 50) value of DTX was 6 nM in human
epidermoid cancer cells (A431),8 and DTX-loaded MPEG-
PDLLA (MPEG-PDLLA-DTX) micelles have entered the
clinical stage in South Korea,9 which could improve the
efficacy of chemotherapy via enhanced permeability and
retention (EPR) effect.10−13 However, it is reported that only
a few drugs could arrive at the tumor site through the EPR
Received: March 13, 2017
Accepted: April 18, 2017
Published: April 18, 2017
© 2017 American Chemical Society
15317
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
Downloaded via BEIJING UNIV OF CHEMICAL TECHNOLOGY on December 17, 2018 at 09:14:26 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. �
ACS Applied Materials & Interfaces
Research Article
effect.14,15 In order to increase the efficiency in chemotherapy,
several synergetic strategies must be designed to further
enhance the antitumor efficiency.
Near-infrared (NIR) responsive photothermal
therapy
(PTT) is an attractive alternative to combine with chemo-
therapy as it could cause membrane damage, cell injury, and
protein denaturation as a systemic effect.16−19 What’s more,
PTT could induce tumor thermal and improve the accumu-
lation of nanodrug in tumor sites.20 Chen et al. in Soochow
University have used an imagable and photothermal HSA-ICG-
PTX nanoparticle to treat subcutaneous and metastatic breast
tumors, and the combination therapy achieved excellent
synergistic therapeutic efficacy.21 To date, a variety of NIR-
responsive nanostructures were used for photothermal therapy,
such as gold nanostar,22 nanocage,23 nanocube,24 nanocluster,25
nanoshell,26 and nanorod.27 Among them, gold nanorod
(GNR) was chosen as an ideal photothermal agent, which
has several advantages, including easy synthesis, small size, and
adjustable aspect ratio to achieve NIR light absorption.28−30
However, in traditional chemotherapy and PTT, chemotherapy
drugs or photothermal agents were delivered to tumor sites
either intratumorally or intravenously, which not only needed a
high dosage of chemotherapy drugs or photothermal agents to
achieve better therapeutic efficacy but also caused serious side
effects and reduced patients’ quality of life.31 Hence, it is urgent
to develop a novel synergetic system of chemotherapy and PTT
to enhance the antitumor efficiency.
Nowadays, a microneedle delivery system is widely used to
deliver drugs,32,33 DNA,34 RNA,35 and vaccines36 as the
microinjection is a rapid, cost-effective, painless, and direct
way for molecule delivery.37,38 Compared with other drug
delivery ways, the solid microneedles could pass through the
cutin layer of skin and deliver drug to the dermis directly, which
could improve the local drug concentration and reduce
systemic side effects. Both Gu39−42 et al. in North Carolina
State University and Chen43−46 et al. in National Cheng Kung
University have made great contributions in the field of
microneedle delivery systems, which proved that microneedles
are ideal vehicles for molecule delivery.
Herein, we developed a novel
synergetic system of
chemotherapy and PTT to treat
the A431 tumor by the
combination of a NIR-responsive PEGylated gold nanorod
(GNR-PEG) coated poly(L-lactide) microneedle (GNR-PEG@
MNs) and DTX-loaded MPEG-PDLLA micelles shown in
Figure 1. This system was composed of biodegradable poly(L-
lactide) microneedles (PLLA MNs), photothermal agent GNR-
PEG, and antitumor nanodrug MPEG-PDLLA-DTX micelles.
PLLA has been approved by the Food and Drug Administration
(FDA) for clinical use which was suitable as a needle material.
GNR-PEG was a harmless photothermal agent that efficiently
converted the absorbed light energy into heat and made the
tumor site thermal.29,30 Meanwhile, MPEG-PDLLA was a safe
nanocarrier, and DTX-loaded MPEG-PDLLA-DTX micelles
were sensitive to A431 cells.8,9 Last but not least, the above-
mentioned systems have not been reported for the combination
of chemotherapy and PTT before. We aimed to use this novel
NIR responsive GNR-PEG@MN which contained only 31.83
± 1.22 μg of GNR-PEG per patch and exhibited excellent
heating efficacy to enhance the antitumor efficiency of low
dosage MPEG-PDLLA-DTX micelles (5 mg/kg).
In this study, we prepared MPEG-PDLLA-DTX micelles and
GNR-PEG via described methods. The GNR-PEG@MNs with
the height of 480 μm were prepared by using NIR-responsive
Figure 1. Schematic illustration of (A) the preparation of PLLA MNs
and GNR-PEG@MNs, (B) the novel synergetic system of chemo-
therapy and photothermal therapy to treat A431 tumors by the
combination of near-infrared responsive GNR-PEG@MNs and
MPEG-PDLLA-DTX micelles. (Step 1: Injected the DTX loaded
micelles; Step 2: After the injection, pressed the GNR-PEG@MNs at
the tumor sites and under 2 W/cm2 irradiation by 808 nm laser within
5 min.)
GNR-PEG adsorbed on PLLA MNs which contained only
31.83 ± 1.22 μg of GNR-PEG per patch. In addition, the GNR-
PEG@MN was characterized in terms of scanning electron
microscope (SEM), energy spectrum (EDS), skin insertion test,
heating transfer experiment, and NIR thermal imaging study.
Finally, in vivo antitumor efficacy of combined chemotherapy
and PTT was carried out, and the combination of MPEG-
PDLLA-DTX micelles and GNR-PEG@MNs was compared
with that of chemotherapy or PTT alone in mice bearing an
A431 tumor. The histopathological and cell proliferation of the
tumors was also observed.
■ RESULTS AND DISCUSSION
Characterization of MPEG-PDLLA. The MPEG-PDLLA
copolymer (Mn = 3765, 2000−1765) was synthesized via ring-
opening method47 by MPEG 2000 and D,L-lactide as described
before48 (Figure S1A in the Supporting Information (SI) shows
the procedure). The characterization of MPEG-PDLLA by 1H
NMR spectra (Varian 400 spectrometer, Varian, USA), Fourier
transform infrared spectroscopy (FTIR, NICOLET 200SXV,
Nicolet, USA), and gel permeation chromatography (GPC,
Agilent 110 HPLC, USA)49,50 confirmed that the MPEG-
PDLLA copolymer was synthesized successfully and the
molecular weight was 3821 with narrow distribution of 1.08.
(Figure S1B−D in the SI)
The DTX-loaded MPEG-PDLLA micelles were prepared via
a thin-film rehydration method.6,9 Dynamic light scattering
15318
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
Research Article
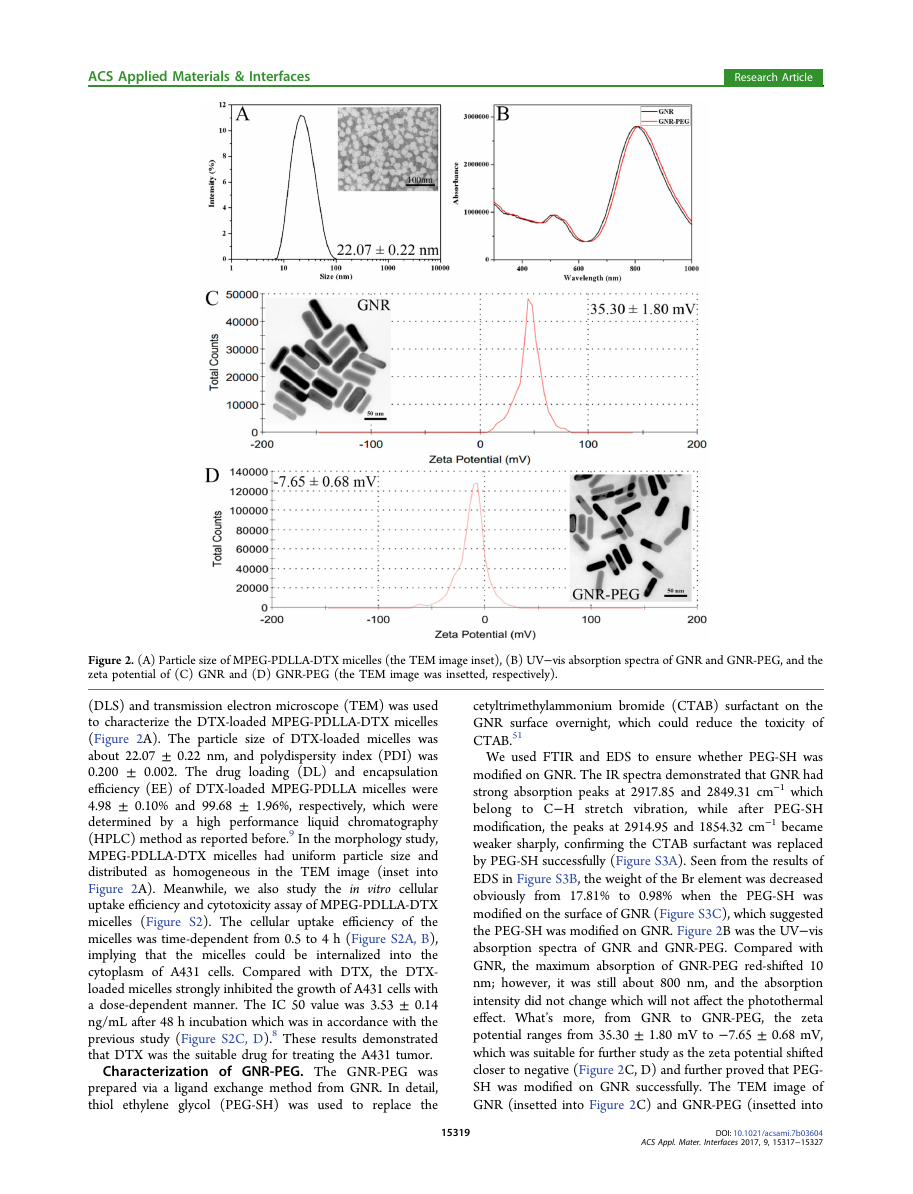
Figure 2. (A) Particle size of MPEG-PDLLA-DTX micelles (the TEM image inset), (B) UV−vis absorption spectra of GNR and GNR-PEG, and the
zeta potential of (C) GNR and (D) GNR-PEG (the TEM image was insetted, respectively).
(DLS) and transmission electron microscope (TEM) was used
to characterize the DTX-loaded MPEG-PDLLA-DTX micelles
(Figure 2A). The particle size of DTX-loaded micelles was
about 22.07 ± 0.22 nm, and polydispersity index (PDI) was
0.200 ± 0.002. The drug loading (DL) and encapsulation
efficiency (EE) of DTX-loaded MPEG-PDLLA micelles were
4.98 ± 0.10% and 99.68 ± 1.96%, respectively, which were
determined by a high performance liquid chromatography
(HPLC) method as reported before.9 In the morphology study,
MPEG-PDLLA-DTX micelles had uniform particle size and
distributed as homogeneous in the TEM image (inset into
Figure 2A). Meanwhile, we also study the in vitro cellular
uptake efficiency and cytotoxicity assay of MPEG-PDLLA-DTX
micelles (Figure S2). The cellular uptake efficiency of the
micelles was time-dependent from 0.5 to 4 h (Figure S2A, B),
implying that
the micelles could be internalized into the
cytoplasm of A431 cells. Compared with DTX, the DTX-
loaded micelles strongly inhibited the growth of A431 cells with
a dose-dependent manner. The IC 50 value was 3.53 ± 0.14
ng/mL after 48 h incubation which was in accordance with the
previous study (Figure S2C, D).8 These results demonstrated
that DTX was the suitable drug for treating the A431 tumor.
Characterization of GNR-PEG. The GNR-PEG was
prepared via a ligand exchange method from GNR. In detail,
thiol ethylene glycol (PEG-SH) was used to replace the
cetyltrimethylammonium bromide (CTAB) surfactant on the
GNR surface overnight, which could reduce the toxicity of
CTAB.51
We used FTIR and EDS to ensure whether PEG-SH was
modified on GNR. The IR spectra demonstrated that GNR had
strong absorption peaks at 2917.85 and 2849.31 cm−1 which
belong to C−H stretch vibration, while after PEG-SH
modification, the peaks at 2914.95 and 1854.32 cm−1 became
weaker sharply, confirming the CTAB surfactant was replaced
by PEG-SH successfully (Figure S3A). Seen from the results of
EDS in Figure S3B, the weight of the Br element was decreased
obviously from 17.81% to 0.98% when the PEG-SH was
modified on the surface of GNR (Figure S3C), which suggested
the PEG-SH was modified on GNR. Figure 2B was the UV−vis
absorption spectra of GNR and GNR-PEG. Compared with
GNR, the maximum absorption of GNR-PEG red-shifted 10
nm; however, it was still about 800 nm, and the absorption
intensity did not change which will not affect the photothermal
effect. What’s more,
the zeta
potential ranges from 35.30 ± 1.80 mV to −7.65 ± 0.68 mV,
which was suitable for further study as the zeta potential shifted
closer to negative (Figure 2C, D) and further proved that PEG-
SH was modified on GNR successfully. The TEM image of
GNR (insetted into Figure 2C) and GNR-PEG (insetted into
from GNR to GNR-PEG,
15319
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
Research Article
Figure 3. (A) Photograph of PLLA MNs (left) and GNR-PEG@MNs (right), SEM image of (B1−B2) PLLA MNs and (C1−C2) GNR-PEG@MNs
(the black square represents the location of GNR-PEG), and the surface elemental content of (B3) PLLA MNs and (C3) GNR-PEG@MNs (B1,C1,
scale bar, 100 μm; B2,C2, scale bar, 10 μm).
Figure 2D) revealed that GNR and GNR-PEG had uniform
length about 50 nm and good dispersion.
Characterization of GNR-PEG@MNs. The NIR-respon-
sive GNR-PEG@MNs were obtained from the modification of
PLLA MNs. First, the PLLA MN was fabricated by melt-
molding PLLA on a polydimethylsiloxane (PDMS) template
and press-molded to obtain arrays of microneedles.34,52 The
NIR-responsive GNR-PEG adsorbed on PLLA MNs via the
layer by layer method,53,54 and then we used scanning electron
microscopy (SEM), energy-dispersive spectroscopy (EDS),
FTIR, UV−vis transmittance spectroscopy, and X-ray photo-
electron spectroscopy (XPS) to characterize the microneedles.
The size of PLLA MNs (left) was 1 cm × 1 cm which consisted
of 400 (20 × 20) microneedle tips. When the GNR-PEG
absorbed on PLLA MNs to get GNR-PEG@MNs (right), the
color of the microneedles turned white to purple (Figure 3A).
What’s more, the micrographs of PLLA MNs and GNR-PEG@
MNs shown in Figure S4 presented the bright-field micro-
graphs of microneedle structures. Figure 3B1 and Figure 3C1
were the SEM image of PLLA MNs and GNR-PEG@MNs, and
the microneedle tips were lined up in order. The base width
the microneedles were 300 and 480 μm,
and height of
respectively. The surface of PLLA MNs was clean and smooth
(Figure 3B2), while the surface of GNR-PEG@MNs had a lot
of rod-shaped objects that were GNR-PEG (Figure 3C2). We
used EDS to analyze the elemental content of PLLA MNs and
GNR-PEG@MNs (Figure 3B3, C3). The results showed that
only C, O elements were on the surface of PLLA MNs, while
the surface of GNR-PEG@MNs had C, O, and Au elements,
demonstrating that the GNR-PEG absorbed on the surface of
PLLA MNs successfully. This is further supported by IR
spectra, UV−vis
transmittance spectra, and XPS results
(Figures S5 and S6). In detail, when polyethylenimine (PEI)
modified PLLA MNs, the absorption peak at 1591.21 cm−1 in
the IR spectra was attributed to νNH stretch vibration, and the
peak disappeared after GNR-PEG absorbed on PEI-modified
PLLA MNs (Figure S5A),
revealing the GNR-PEG was
absorbed on PLLA MNs successfully. What’s more,
the
transmittance measured via UV−vis transmittance spectroscopy
declined from 81% to 64% as the modification carried on
(Figure S5B), which also demonstrated that we obtained GNR-
PEG@MNs via a layer by layer method. The surface atomic
composition of GNR-PEG@MNs was identified by XPS
analysis (Figure S6). Compared with PLLA MNs, GNR-
PEG@MNs had a Au signal, indicating that the GNR-PEG was
successfully absorbed on PLLA MNs.
In Vitro Skin Insertion Test. Skin insertion ability is the
key factor to overcome the skin resistance for drug delivery.43,44
The trypan blue staining method was used to confirm whether
the GNR-PEG@MNs could insert into the skin completely.
After GNR-PEG@MNs inserted into mice skin for 5 min, the
skin treated with trypan blue showed consistent microneedle
insertion (Figure 4A). The surface of the skin revealed rows of
Figure 4. (A) Photograph, (B) optical micrograph (scale bar, 200
μm), and (C) histological sections (scale bar, 20 μm) of mice skin
stained with trypan blue after GNR-PEG@MN application (the black
arrow represents the microneedle puncture sites) and H&E-stained
mice skin sections (D) after GNR-PEG@MN application (scale bar,
20 μm) (the black dotted line represents the microneedle puncture
sites) and (E) 12 h after GNR-PEG@MN application (scale bar, 20
μm).
15320
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
Research Article
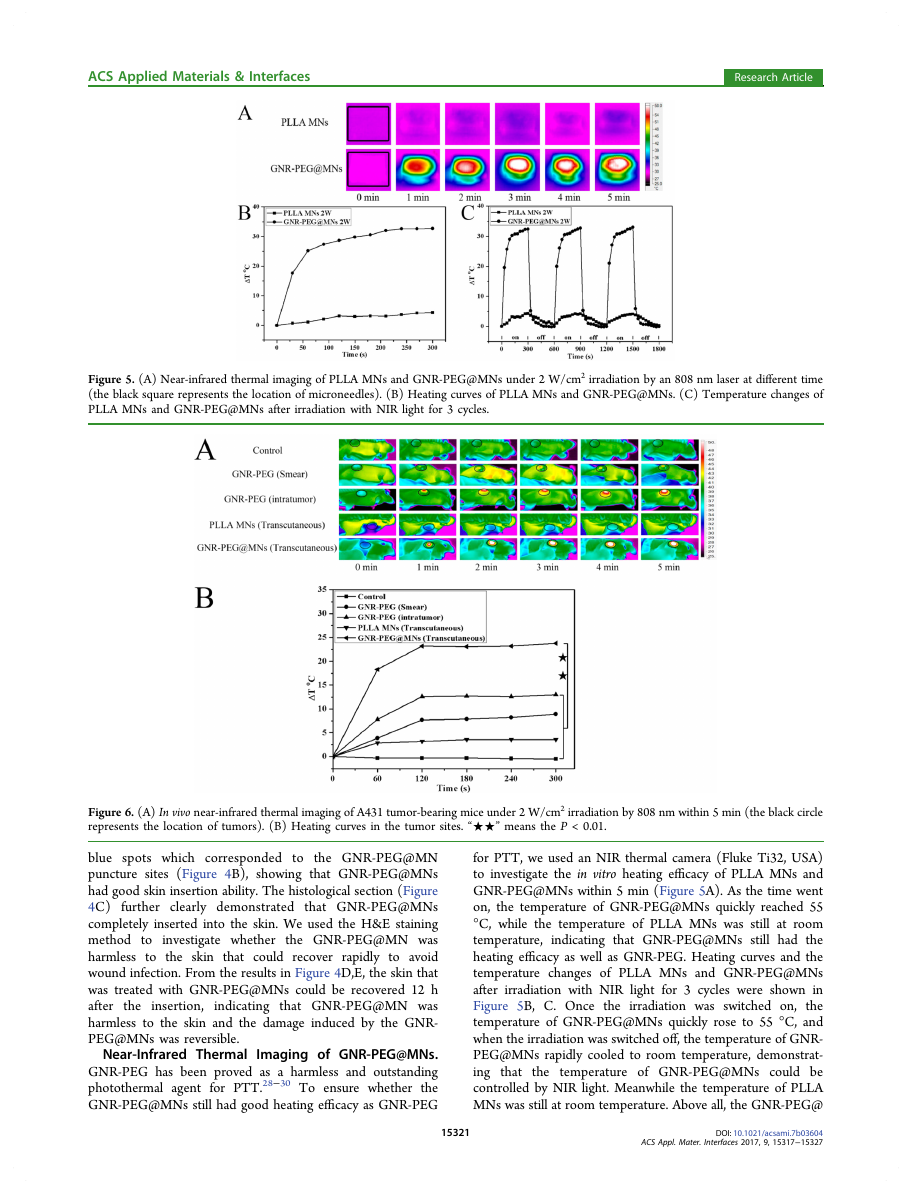
Figure 5. (A) Near-infrared thermal imaging of PLLA MNs and GNR-PEG@MNs under 2 W/cm2 irradiation by an 808 nm laser at different time
(the black square represents the location of microneedles). (B) Heating curves of PLLA MNs and GNR-PEG@MNs. (C) Temperature changes of
PLLA MNs and GNR-PEG@MNs after irradiation with NIR light for 3 cycles.
Figure 6. (A) In vivo near-infrared thermal imaging of A431 tumor-bearing mice under 2 W/cm2 irradiation by 808 nm within 5 min (the black circle
represents the location of tumors). (B) Heating curves in the tumor sites. “★★” means the P < 0.01.
blue spots which corresponded to the GNR-PEG@MN
puncture sites (Figure 4B), showing that GNR-PEG@MNs
had good skin insertion ability. The histological section (Figure
4C) further clearly demonstrated that GNR-PEG@MNs
completely inserted into the skin. We used the H&E staining
method to investigate whether
the GNR-PEG@MN was
harmless to the skin that could recover rapidly to avoid
wound infection. From the results in Figure 4D,E, the skin that
was treated with GNR-PEG@MNs could be recovered 12 h
after
indicating that GNR-PEG@MN was
harmless to the skin and the damage induced by the GNR-
PEG@MNs was reversible.
Near-Infrared Thermal
Imaging of GNR-PEG@MNs.
GNR-PEG has been proved as a harmless and outstanding
photothermal agent for PTT.28−30 To ensure whether the
GNR-PEG@MNs still had good heating efficacy as GNR-PEG
the insertion,
for PTT, we used an NIR thermal camera (Fluke Ti32, USA)
to investigate the in vitro heating efficacy of PLLA MNs and
GNR-PEG@MNs within 5 min (Figure 5A). As the time went
on, the temperature of GNR-PEG@MNs quickly reached 55
°C, while the temperature of PLLA MNs was still at room
temperature,
indicating that GNR-PEG@MNs still had the
heating efficacy as well as GNR-PEG. Heating curves and the
temperature changes of PLLA MNs and GNR-PEG@MNs
after irradiation with NIR light for 3 cycles were shown in
Figure 5B, C. Once the irradiation was switched on,
the
temperature of GNR-PEG@MNs quickly rose to 55 °C, and
when the irradiation was switched off, the temperature of GNR-
PEG@MNs rapidly cooled to room temperature, demonstrat-
ing that
the temperature of GNR-PEG@MNs could be
controlled by NIR light. Meanwhile the temperature of PLLA
MNs was still at room temperature. Above all, the GNR-PEG@
15321
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
Research Article
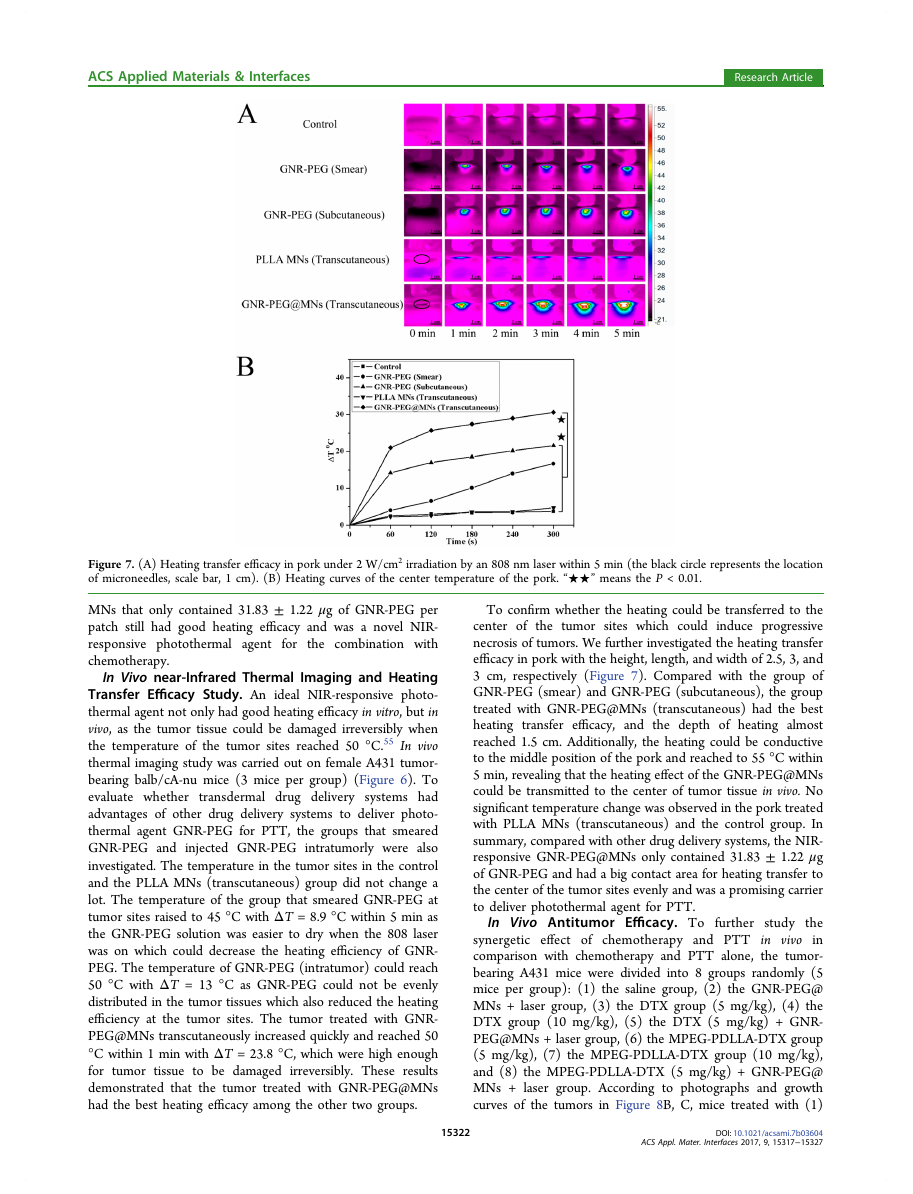
Figure 7. (A) Heating transfer efficacy in pork under 2 W/cm2 irradiation by an 808 nm laser within 5 min (the black circle represents the location
of microneedles, scale bar, 1 cm). (B) Heating curves of the center temperature of the pork. “★★” means the P < 0.01.
for
MNs that only contained 31.83 ± 1.22 μg of GNR-PEG per
patch still had good heating efficacy and was a novel NIR-
responsive photothermal agent
the combination with
chemotherapy.
In Vivo near-Infrared Thermal Imaging and Heating
Transfer Efficacy Study. An ideal NIR-responsive photo-
thermal agent not only had good heating efficacy in vitro, but in
vivo, as the tumor tissue could be damaged irreversibly when
the temperature of the tumor sites reached 50 °C.55 In vivo
thermal imaging study was carried out on female A431 tumor-
bearing balb/cA-nu mice (3 mice per group) (Figure 6). To
evaluate whether
transdermal drug delivery systems had
advantages of other drug delivery systems to deliver photo-
thermal agent GNR-PEG for PTT, the groups that smeared
GNR-PEG and injected GNR-PEG intratumorly were also
investigated. The temperature in the tumor sites in the control
and the PLLA MNs (transcutaneous) group did not change a
lot. The temperature of the group that smeared GNR-PEG at
tumor sites raised to 45 °C with ΔT = 8.9 °C within 5 min as
the GNR-PEG solution was easier to dry when the 808 laser
was on which could decrease the heating efficiency of GNR-
PEG. The temperature of GNR-PEG (intratumor) could reach
50 °C with ΔT = 13 °C as GNR-PEG could not be evenly
distributed in the tumor tissues which also reduced the heating
efficiency at the tumor sites. The tumor treated with GNR-
PEG@MNs transcutaneously increased quickly and reached 50
°C within 1 min with ΔT = 23.8 °C, which were high enough
for tumor tissue to be damaged irreversibly. These results
demonstrated that the tumor treated with GNR-PEG@MNs
had the best heating efficacy among the other two groups.
To confirm whether the heating could be transferred to the
center of
the tumor sites which could induce progressive
necrosis of tumors. We further investigated the heating transfer
efficacy in pork with the height, length, and width of 2.5, 3, and
3 cm, respectively (Figure 7). Compared with the group of
GNR-PEG (smear) and GNR-PEG (subcutaneous), the group
treated with GNR-PEG@MNs (transcutaneous) had the best
heating transfer efficacy, and the depth of heating almost
reached 1.5 cm. Additionally, the heating could be conductive
to the middle position of the pork and reached to 55 °C within
5 min, revealing that the heating effect of the GNR-PEG@MNs
could be transmitted to the center of tumor tissue in vivo. No
significant temperature change was observed in the pork treated
with PLLA MNs (transcutaneous) and the control group. In
summary, compared with other drug delivery systems, the NIR-
responsive GNR-PEG@MNs only contained 31.83 ± 1.22 μg
of GNR-PEG and had a big contact area for heating transfer to
the center of the tumor sites evenly and was a promising carrier
to deliver photothermal agent for PTT.
In Vivo Antitumor Efficacy. To further
study the
synergetic effect of chemotherapy and PTT in vivo in
comparison with chemotherapy and PTT alone, the tumor-
bearing A431 mice were divided into 8 groups randomly (5
mice per group): (1) the saline group, (2) the GNR-PEG@
MNs + laser group, (3) the DTX group (5 mg/kg), (4) the
DTX group (10 mg/kg), (5) the DTX (5 mg/kg) + GNR-
PEG@MNs + laser group, (6) the MPEG-PDLLA-DTX group
(5 mg/kg), (7) the MPEG-PDLLA-DTX group (10 mg/kg),
and (8) the MPEG-PDLLA-DTX (5 mg/kg) + GNR-PEG@
MNs + laser group. According to photographs and growth
curves of the tumors in Figure 8B, C, mice treated with (1)
15322
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
Research Article
Figure 8. (A) Schematic illustration of in vivo antitumor efficacy of the combination of GNR-PEG@MNs and MPEG-PDLLA-DTX micelles. (B)
Representative photos of mice bearing A431 tumors after treatment for 7, 14, 21, 28, and 35 days (the black circle represents the location of tumors).
(C) Growth curves and (D) body weight of the mice in each group. (E) Photograph and (F) weight of subcutaneous tumors in each group (the
black circle represents the location of cured tumors). Data are represented as the mean ± standard deviation (n = 5). “★★” and “★” means the P <
0.01 and P < 0.05. (1. Saline, 2. GNR-PEG@MNs + laser, 3. DTX (5 mg/kg), 4. DTX (10 mg/kg), 5. DTX (5 mg/kg) + GNR-PEG@MNs + laser,
6. MPEG-PDLLA-DTX (5 mg/kg), 7. MPEG-PDLLA-DTX (10 mg/kg), 8. MPEG-PDLLA-DTX (5 mg/kg) + GNR-PEG@MNs + laser.)
Figure 9. (A) Representative H&E stained images (scale bar, 20 μm), (B) Ki-67 immune histochemical images (scale bar, 20 μm), and (C) Ki-67 LI
of tumors in each group. “★★” means the P < 0.01. (1. Saline, 2. GNR-PEG@MNs + laser, 3. DTX (5 mg/kg), 4. DTX (10 mg/kg), 5. DTX (5 mg/
kg) + GNR-PEG@MNs + laser, 6. MPEG-PDLLA-DTX (5 mg/kg), 7. MPEG-PDLLA-DTX (10 mg/kg), 8. MPEG-PDLLA-DTX (5 mg/kg) +
GNR-PEG@MNs + laser.)
saline had a rapid tumor growth, and then was the group of (2)
GNR-PEG@MNs + laser, indicating that the GNR-PEG@MNs
+ laser group could inhibit tumor slightly which was due to
PTT. Additionally, the (5) DTX (5 mg/kg) + GNR-PEG@
MNs + laser group had better inhibit ability than (3) DTX (5
mg/kg) and (4) DTX (10 mg/kg) groups in the early times,
while the tumors had a recurrence at day 25 after the treatment,
implying that
the GNR-PEG@MNs could enhance the
antitumor ability of DTX, but the low dosage of DTX could
not inhibit tumor growth for a long time. Compared to the
DTX group with DTX-loaded MPEG-PDLLA micelles at the
same dosage, the MPEG-PDLLA-DTX micelles group had
better antitumor ability than the DTX group, which was
consistent with the previously reported study.56,57 Whether the
DTX group or DTX-loaded MPEG-PDLLA micelles group, the
antitumor ability of high dose (10 mg/kg) was better than the
low dose (5 mg/kg) group. It was noted that the (8) MPEG-
PDLLA-DTX (5 mg/kg) + GNR-PEG@MNs + laser group
15323
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�
ACS Applied Materials & Interfaces
had a more rapid tumor volume reduction than the group of
(7) MPEG-PDLLA-DTX (10 mg/kg), and all mice were cured
within 14 days after the treatment. In detail, after the combined
treatment,
the tumors were reduced rapidly and gradually
replaced by a scab. Then the scab faded, and the skin was cured
at day 20. Notably, no tumor recurrence was observed up to
now, demonstrating that the GNR-PEG@MNs could enhance
the antitumor ability of MPEG-PDLLA-DTX micelles and
reduce the dosage of DTX. Moreover, the photograph of the
tumor in the mice (Figure 8E) was consistent with Figure 8B,
C. As shown in Figure 8D, the body weight of the mice did not
change a lot in the treatment groups. The statistic data of the
tumor weight was presented in Figure 8F. What’s more, the
major tissues were collected for histopathology study (Figure
S7), and no lesions or inflammation was found in the hearts,
livers, spleens, and kidneys tissues. For lung tissues,
it was
obvious to see congestion, necrosis, and alveolar deformation in
(1) saline and (2) GNR-PEG@MNs + laser group. These
results demonstrated that the GNR-PEG@MN was a safer
carrier for PTT and could improve the antitumor ability of
MPEG-PDLLA-DTX micelles.
In short, the synergetic effect of the combination of low
dosage MPEG-PDLLA-DTX micelles (5 mg/kg) and GNR-
PEG@MNs completely eradicated the A431 tumor without
recurrence in vivo. The photothermal agent GNR-PEG@MNs
was inserted into the tumor site transcutaneously which was
harmless to the skin and minimized the uncomfortable feeling.
With the combination of GNR-PEG@MNs, the dosage of
MPEG-PDLLA-DTX micelles reduced to 5 mg/kg, which
could reduce the occurrence of serious side effects. Compared
with other chemo−photothermal
therapy systems,12,58 this
synergetic system is expected to have great potential in clinical
translation for human epidermoid cancer therapy.
Tumor cells Histopathological and Proliferation
Study. Inspired by the results of in vivo antitumor efficacy,
we explored the tumor cells histopathology and proliferation (3
mice per group). Histopathological study of tumor tissues
(Figure 9A) revealed that although apoptosis and necroptosis
occurred in the tumor site of the (2) GNR-PEG@MNs + laser
group there were still some surviving tumor cells in the tumor
site. Compared with the group of (3) DTX (5 mg/kg), (4)
DTX (10 mg/kg), and (5) DTX (5 mg/kg) + GNR-PEG@
MNs + laser, more tumor cells disappeared in the tumor site in
the (7) MPEG-PDLLA-DTX (10 mg/kg) micelles group and
(8) MPEG-PDLLA-DTX (5 mg/kg) + GNR-PEG@MNs +
laser group, which further indicated that micelles have good
antitumor ability and the GNR-PEG@MNs played an
important
role in enhancing the antitumor efficacy and
reducing the dosage of drug for cancer therapy.
The proliferation of the tumor cell was investigated by Ki-67
immune histochemical staining (Figure 9B). The saline group
has the most Ki-67 immunoreactivity in the treated groups.
Compared with the DTX and the MPEG-PDLLA-DTX group,
the micelles group had less Ki-67 positive cells in tumor tissues
at the same dose, indicating that the micelles could significantly
inhibit tumor cell proliferation. What’s more, the groups of (5)
DTX (5 mg/kg) + GNR-PEG@MNs + laser and (8) MPEG-
PDLLA-DTX (5 mg/kg) + GNR-PEG@MNs + laser group
had better ability of inhibiting tumor cell proliferation than (3)
DTX (5 mg/kg) and (6) MPEG-PDLLA-DTX (5 mg/kg)
micelles groups, demonstrating that
the GNR-PEG@MNs
could enhance the antitumor ability of MPEG-PDLLA-DTX
under the irradiation of 808 nm laser and reduce the dosage of
Research Article
the drug. The quantitative data of the proliferation of tumor
cells are shown in Figure 9C.
■ CONCLUSIONS
In summary, we successfully prepared MPEG-PDLLA-DTX
micelles, GNR-PEG, and PLLA MNs. Then we used the layer
by layer method to achieve GNR-PEG@MNs. The GNR-
PEG@MNs had good skin insert ability and were harmless to
the skin. In addition, the GNR-PEG@MNs had the heating
efficacy as well as GNR-PEG, and the temperature of GNR-
PEG@MNs could be controlled by NIR light. The in vivo NIR
thermal imaging and heating transfer efficacy study proved that
the heating effect of the GNR-PEG@MNs could be transmitted
to the middle of the tumor tissue and reach 50 °C. The
combination of GNR-PEG@MNs and low dosage of MPEG-
PDLLA-DTX micelles (5 mg/kg) efficiently inhibited the
tumor growth and cured all mice without
recurrence.
Therefore, this novel NIR-responsive GNR-PEG@MN could
be a promising strategy to enhance the antitumor effect of the
chemotherapy and is expected to have a great potential
in
clinical translation for human epidermoid cancer therapy.
■ MATERIALS AND METHODS
Preparation and Characterization of DTX-Loaded Micelles.
In this study, we prepared DTX-loaded MPEG-PDLLA micelles
according to a previously published method.6,9 In detail, 5 mg of DTX
and 95 mg of MPEG-PDLLA were dissolved together in appropriate
anhydrous ethanol. Then the ethanol was removed in vacuum at 37 °C
by a rotary evaporator. Finally, the MPEG-PDLLA-DTX micelles were
prepared by adding 5 mL of deionized water at 60 °C and filtered with
a 220 nm syringe filter. The DL and EE of DTX-loaded MPEG-
PDLLA micelles were determined by the HPLC (Agilent 1260 HPLC,
USA) method as reported before.9 DLS (Nano-ZS90, UK) and TEM
(H-6009IV,
Japan) were used to characterize the DTX-loaded
micelles. The cellular uptake efficiency and in vitro cytotoxicity of
DTX and MPEG-PDLLA-DTX micelles were carried out on A431 cell
lines.
Preparation and Characterization of GNR-PEG. The GNR was
synthesized through a seed-mediated method described before.19 The
GNR-PEG was synthesized via a ligand exchange method.51 Briefly, 1
mL of GNR (1 mg/mL) was reacted with 20 mg of PEG-SH dissolved
in 19 mL of deionized water and stirred at 25 °C. After reaction for the
night, the GNR-PEG was centrifuged at 12 000 rpm for 15 min, and
then the the precipitates were collected and dispersed in deionized
water. FTIR and EDS (JSM-7500F, JEOL, Japan) were used to
confirm whether PEG-SH was modified on GNR successfully. The
morphology, zeta potential distribution, and absorption spectra of
GNR and GNR-PEG were measured by TEM, DLS, and UV−vis
absorption spectrometry (PE, USA), respectively.
Preparation of PLLA MNs. The microneedle master structure
which was made from a polydimethylsiloxane (PDMS) template was
supplied by Jianghan University. The PLLA MNs were prepared as
follows. First, the dried PLLA was placed on a PDMS mold and then
press-molded at 170 °C for 15 min on the as-prepared PDMS mold to
obtain arrays of PLLA MNs.
Preparation and Characterization of GNR-PEG@MNs. The
layer by layer method53,54 was used to prepare GNR-PEG@MNs.
Briefly, the PLLA MNs were put into 50 mL of PEI solution (0.5 mg/
mL) for 2 h to get positive charge. Then pure water was used to wash
the PLLA MNs and blown dry by nitrogen. In addition, PLLA MNs
with positive charge were put into GNR-PEG solution (0.5 mg/mL)
which had negative charge for 2 h. At last, pure water was used to wash
them, and they were blown dry by nitrogen to get GNR-PEG@MNs
for which the gold content was 31.83 ± 1.22 μg per patch measured by
inductively coupled plasma-atomic emission spectrometry (ICP-AES).
FTIR and UV−vis transmittance spectroscopy (PE, USA) were used
to ensure whether GNR-PEG absorbed on PLLA MNs to achieve
15324
DOI: 10.1021/acsami.7b03604
ACS Appl. Mater. Interfaces 2017, 9, 15317−15327
�













 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc