中国科技论文在线
http://www.paper.edu.cn
Vacuum Ultraviolet Excited Photoluminescence of
CaWO4-Based White Spherical Nanophosphor with
Codoping of Dy3+ and Na+#
Wang Zhaofeng, Wang Yuhua
(Department of Materials Science, School of Physical Science and Technology, Lanzhou
University, LanZhou 730000)
Abstract: In this paper, Dy3+, Na+ codoped calcium tungstate (CaWO4: Dy3+, Na+) nanophosphors
were synthesized by hydrothermal reaction and their photoluminescence properties under vacuum
ultraviolet (VUV) excitation were investigated. The morphology of the as-prepared sample was
confirmed to be nanosphere with the diameter of 70 nm by SEM and TEM. The excitation spectrum
showed that CaWO4: Dy3+, Na+ nanophosphor has strong absorption in VUV region and energy
transfer could be easily occurred from the host to the energy levels of Dy3+. When excited by 147 nm,
2- besides the f-f transition of Dy3+
the nanophosphor exhibited the characteristic emission of WO4
leading to an optimal pure white light. All of the results indicate that CaWO4: Dy3+, Na+ nanophosphor
has excellent white photoluminescence properties in VUV region and potential applications in
miniature illumination device.
Keywords: Photoluminescence; Nanophosphor; VUV
5
10
15
20
0 Introduction
25
30
35
40
Recently, intense research activity has been focused on nano-sized phosphor regarding its
unique electrical and optical characteristics compared with the bulk phosphor. These natures are
mainly caused by quantum effects which are derived from the decreasing number of allowable
quantum states in the small particles. For example, nanophosphor represents higher quantum
efficiency, longer lifetime and higher quenching concentration than those of the bulk material. In
addition, nanophosphor also exhibits some advantages on the aspect of the application that they
were specially employed to acquire miniature luminescent device and high resolution display
equipment [1-5].
Luminescent materials doped with Dy3+ have attracted much attention for the applications in
light-emitting diodes (LEDs) and non-mercury fluorescent lamps. The luminescence of Dy3+ is
mainly composed of two intrinsic transitions, that is, the blue emission (4F9/2→6H15/2
) at 470~500
nm and the yellow emission (4F9/2→6H13/2
) at 560~600 nm. Therefore, the combined color could
be white, but it is often apt to be yellowish due to the lack of blue component [6-8]. Some reports
have noticed that pure white light of Dy3+ could be obtained by adjusting its doping concentration
since the yellow emission of Dy3+ is sensitive to the lattice environment [9-11]. However, this
approach is still very difficult to give attention to both of its emission intensity and color purity.
It is well known that calcium tungstate (CaWO4; CWO) of scheelite-like structure is an
excellent blue-emitting phosphor by the irradiation of ultraviolet (UV) light [12]. Besides, the
2- group in CaWO4 phosphor could also absorb vacuum ultraviolet (VUV) photons to
WO4
generate blue luminescence [13, 14]. Based on these points, it is possible to generate pure white
light through a strategy of combining the intrinsic luminescence of Dy3+ and CaWO4
with
appropriate ratio, as the shortage of the blue emission of single Dy3+ would be effectively
Foundations: This work was supported by the National Natural Science Foundation of China (No. 10874061), the
Research Fund for the Doctoral Program of Higher Education (No. 200807300010) and the National Science
Foundation for Distinguished Young Scholars (No. 50925206).
Brief author introduction:Wang Zhaofeng,(1986-). Main research area is nanoscaled luminescent materials.
Communication author:Wang Yuhua, Professor of Lanzhou University. Research area is inorganic luminescent
materials
- 1 -
�
45
50
55
60
65
70
中国科技论文在线
compensated by the blue-emitting host. To the best of our knowledge, the photoluminescence of
CaWO4: Dy3+ in UV region (200 ≤ λem ≤ 400 nm) has been investigated by the research of Y. Su et
al. [15], but that in VUV region (λem < 200nm, energy > 50,000cm-1) have not been involved in
any other reference so far.
http://www.paper.edu.cn
Hydrothermal synthesis is an effective approach to acquire nano-materials due to its low
crystallization temperature and controllable morphology of products. Furthermore, high degree of
precipitation in hydrothermal process could reduce the content of metal ions in effluents which is
beneficial to environmental protection [16]. For all the reasons above, we synthesized CWO: Dy3+,
Na+ nanophosphors (Na+ is the charge compensator) by hydrothermal technique and first explored
the luminescent properties of CaWO4: Dy3+ under VUV irradiation including the possible
luminescent mechanism.
1 Experimental details
1.1 Synthesis
The nanophosphors were achieved through hydrothermal processes with the addition of
surfactant CTAB. First, stoichiometric Na2WO4٠2H2O, CaCl2, Dy(NO3)3 (Dy2O3 was dissolved by
nitric acid) and CTAB were dissolved in 25 mL deionized water at the same time. Then the
solution was ceaselessly stirred for 1 h at room temperature. The resulting precursor suspension
was then transferred into a Teflon-lined stainless steel autoclave and treated at 150 oC for 12 h.
After reaction, the autoclave was cooled to room temperature naturally and the products were
collected by centrifugation, washing three times with deionized water and ethanol. After dried in
air at 60 oC for 12 h, the final products were obtained.
1.2 Characterization
Crystal phase of the obtained materials were determined by powder X-ray diffraction (XRD,
Rigaku D/MAX - 2400 X-ray diffractometer with Ni-filtered Cu Kα radiation). The morphology of
the powders was examined by the Transmission Electron Microscopy (TEM) with a Hitachi H-800
transmission electron microscope using an accelerating voltage of 100 kV and field emission
scanning electron microscopy (FESEM; Hitachi, S-4800). The emission, excitation spectra and
decay curves were measured by FLS920T fluorescence spectrophotometer. All measurements
were carried out at room temperature.
2 Results and Discussion
- 2 -
�
中国科技论文在线
http://www.paper.edu.cn
75
80
85
90
95
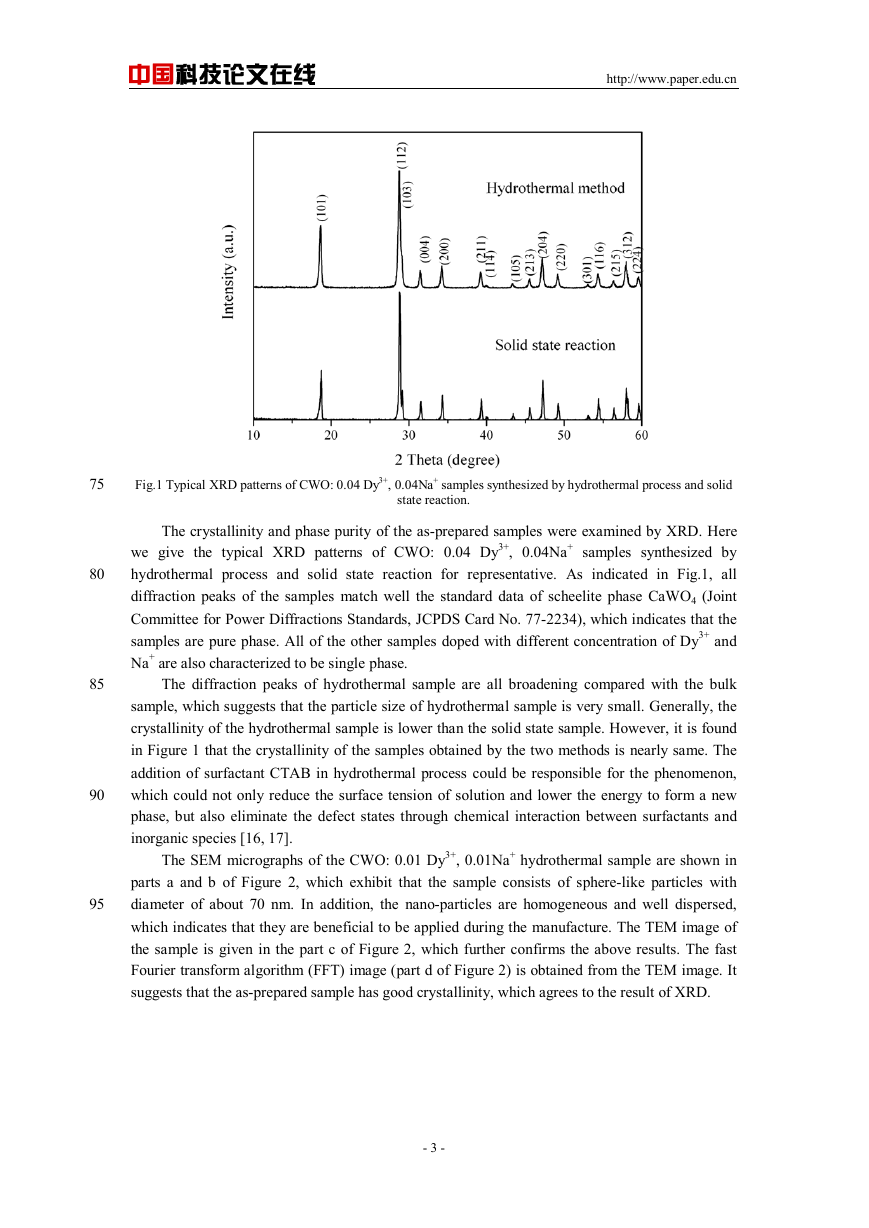
Fig.1 Typical XRD patterns of CWO: 0.04 Dy3+, 0.04Na+ samples synthesized by hydrothermal process and solid
state reaction.
The crystallinity and phase purity of the as-prepared samples were examined by XRD. Here
we give the typical XRD patterns of CWO: 0.04 Dy3+, 0.04Na+ samples synthesized by
hydrothermal process and solid state reaction for representative. As indicated in Fig.1, all
diffraction peaks of the samples match well the standard data of scheelite phase CaWO4 (Joint
Committee for Power Diffractions Standards, JCPDS Card No. 77-2234), which indicates that the
samples are pure phase. All of the other samples doped with different concentration of Dy3+ and
Na+ are also characterized to be single phase.
The diffraction peaks of hydrothermal sample are all broadening compared with the bulk
sample, which suggests that the particle size of hydrothermal sample is very small. Generally, the
crystallinity of the hydrothermal sample is lower than the solid state sample. However, it is found
in Figure 1 that the crystallinity of the samples obtained by the two methods is nearly same. The
addition of surfactant CTAB in hydrothermal process could be responsible for the phenomenon,
which could not only reduce the surface tension of solution and lower the energy to form a new
phase, but also eliminate the defect states through chemical interaction between surfactants and
inorganic species [16, 17].
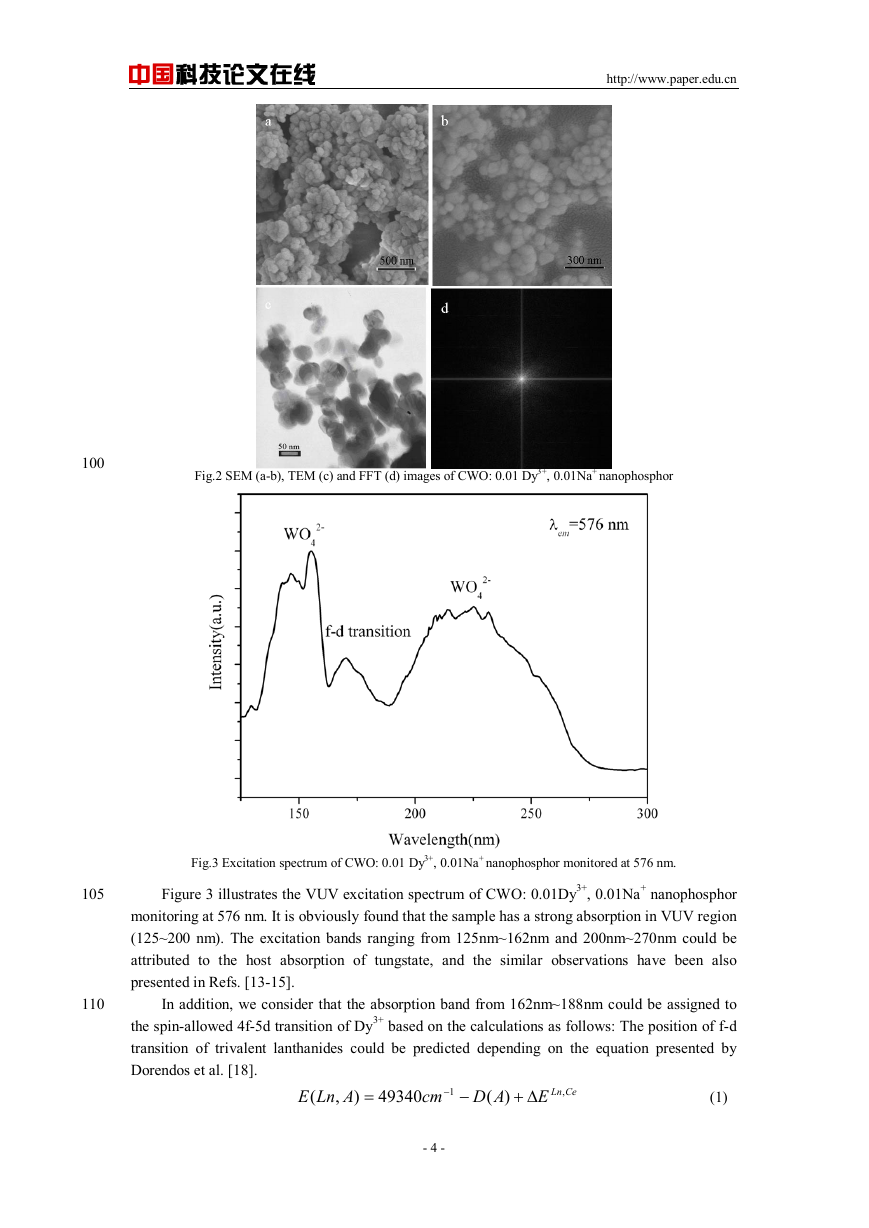
The SEM micrographs of the CWO: 0.01 Dy3+, 0.01Na+ hydrothermal sample are shown in
parts a and b of Figure 2, which exhibit that the sample consists of sphere-like particles with
diameter of about 70 nm. In addition, the nano-particles are homogeneous and well dispersed,
which indicates that they are beneficial to be applied during the manufacture. The TEM image of
the sample is given in the part c of Figure 2, which further confirms the above results. The fast
Fourier transform algorithm (FFT) image (part d of Figure 2) is obtained from the TEM image. It
suggests that the as-prepared sample has good crystallinity, which agrees to the result of XRD.
- 3 -
�
中国科技论文在线
http://www.paper.edu.cn
100
Fig.2 SEM (a-b), TEM (c) and FFT (d) images of CWO: 0.01 Dy3+, 0.01Na+ nanophosphor
105
110
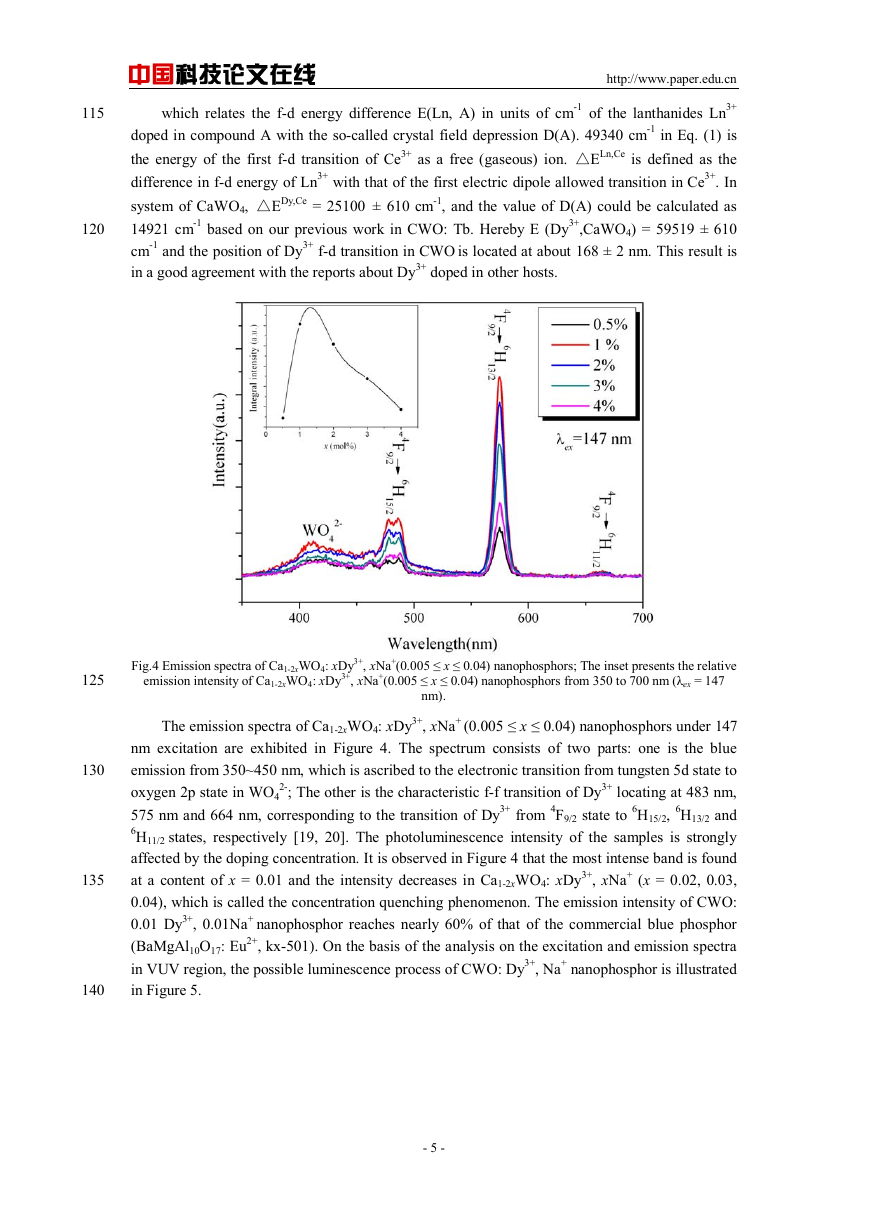
Fig.3 Excitation spectrum of CWO: 0.01 Dy3+, 0.01Na+ nanophosphor monitored at 576 nm.
Figure 3 illustrates the VUV excitation spectrum of CWO: 0.01Dy3+, 0.01Na+ nanophosphor
monitoring at 576 nm. It is obviously found that the sample has a strong absorption in VUV region
(125~200 nm). The excitation bands ranging from 125nm~162nm and 200nm~270nm could be
attributed to the host absorption of tungstate, and the similar observations have been also
presented in Refs. [13-15].
In addition, we consider that the absorption band from 162nm~188nm could be assigned to
the spin-allowed 4f-5d transition of Dy3+ based on the calculations as follows: The position of f-d
transition of trivalent lanthanides could be predicted depending on the equation presented by
Dorendos et al. [18].
ALnE
)
49340
AD
)
(
LnE
Δ+
(1)
−
1
cm
−
,
Ce
(
,
=
- 4 -
�
中国科技论文在线
http://www.paper.edu.cn
115
120
which relates the f-d energy difference E(Ln, A) in units of cm-1 of the lanthanides Ln3+
doped in compound A with the so-called crystal field depression D(A). 49340 cm-1 in Eq. (1) is
the energy of the first f-d transition of Ce3+ as a free (gaseous) ion. △ELn,Ce is defined as the
difference in f-d energy of Ln3+ with that of the first electric dipole allowed transition in Ce3+. In
system of CaWO4, △EDy,Ce = 25100 ± 610 cm-1, and the value of D(A) could be calculated as
14921 cm-1 based on our previous work in CWO: Tb. Hereby E (Dy3+,CaWO4) = 59519 ± 610
cm-1 and the position of Dy3+ f-d transition in CWO is located at about 168 ± 2 nm. This result is
in a good agreement with the reports about Dy3+ doped in other hosts.
125
130
135
140
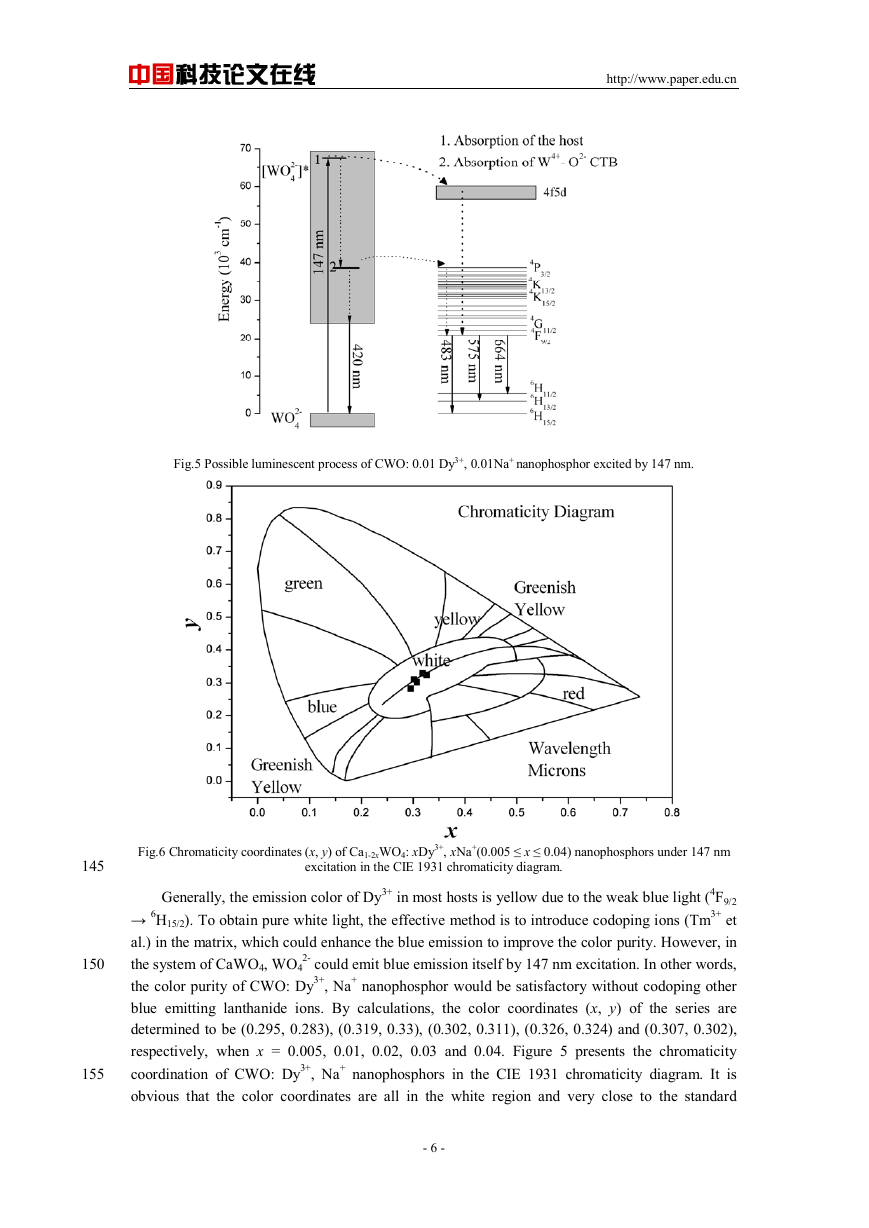
Fig.4 Emission spectra of Ca1-2xWO4: xDy3+, xNa+(0.005 ≤ x ≤ 0.04) nanophosphors; The inset presents the relative
emission intensity of Ca1-2xWO4: xDy3+, xNa+(0.005 ≤ x ≤ 0.04) nanophosphors from 350 to 700 nm (λex = 147
The emission spectra of Ca1-2xWO4: xDy3+, xNa+ (0.005 ≤ x ≤ 0.04) nanophosphors under 147
nm excitation are exhibited in Figure 4. The spectrum consists of two parts: one is the blue
emission from 350~450 nm, which is ascribed to the electronic transition from tungsten 5d state to
2-; The other is the characteristic f-f transition of Dy3+ locating at 483 nm,
oxygen 2p state in WO4
575 nm and 664 nm, corresponding to the transition of Dy3+ from 4F9/2 state to 6H15/2, 6H13/2 and
6H11/2 states, respectively [19, 20]. The photoluminescence intensity of the samples is strongly
affected by the doping concentration. It is observed in Figure 4 that the most intense band is found
at a content of x = 0.01 and the intensity decreases in Ca1-2xWO4: xDy3+, xNa+ (x = 0.02, 0.03,
0.04), which is called the concentration quenching phenomenon. The emission intensity of CWO:
0.01 Dy3+, 0.01Na+ nanophosphor reaches nearly 60% of that of the commercial blue phosphor
(BaMgAl10O17: Eu2+, kx-501). On the basis of the analysis on the excitation and emission spectra
in VUV region, the possible luminescence process of CWO: Dy3+, Na+ nanophosphor is illustrated
in Figure 5.
nm).
- 5 -
�
中国科技论文在线
http://www.paper.edu.cn
Fig.5 Possible luminescent process of CWO: 0.01 Dy3+, 0.01Na+ nanophosphor excited by 147 nm.
145
150
155
Fig.6 Chromaticity coordinates (x, y) of Ca1-2xWO4: xDy3+, xNa+(0.005 ≤ x ≤ 0.04) nanophosphors under 147 nm
excitation in the CIE 1931 chromaticity diagram.
Generally, the emission color of Dy3+ in most hosts is yellow due to the weak blue light (4F9/2
→ 6H15/2). To obtain pure white light, the effective method is to introduce codoping ions (Tm3+ et
al.) in the matrix, which could enhance the blue emission to improve the color purity. However, in
2- could emit blue emission itself by 147 nm excitation. In other words,
the system of CaWO4, WO4
the color purity of CWO: Dy3+, Na+ nanophosphor would be satisfactory without codoping other
blue emitting lanthanide ions. By calculations, the color coordinates (x, y) of the series are
determined to be (0.295, 0.283), (0.319, 0.33), (0.302, 0.311), (0.326, 0.324) and (0.307, 0.302),
respectively, when x = 0.005, 0.01, 0.02, 0.03 and 0.04. Figure 5 presents the chromaticity
coordination of CWO: Dy3+, Na+ nanophosphors in the CIE 1931 chromaticity diagram. It is
obvious that the color coordinates are all in the white region and very close to the standard
- 6 -
�
中国科技论文在线
coordinates (0.33, 0.33). Combining the discussion of the emission intensity and color purity, the
optimum nanoscaled sample is determined to be CWO: 0.01Dy3+, 0.01Na+.
3 Conclusion
http://www.paper.edu.cn
160
165
170
175
180
185
190
195
200
205
210
In
this paper, white-emitting nanophosphors CWO: Dy3+, Na+ were prepared by
hydrothermal reaction and comprehensively discussed in VUV region. The nanophosphors
showed strong absorption in VUV region and the possible luminescent mechanism was concluded.
The optimum nanophosphor (CWO: 0.01 Dy3+, 0.01Na+) emitted strong and pure white light when
2- and the
excited by 147 nm due to the effective combination of the blue light (~ 436 nm) of WO4
characteristic emission of Dy3+ (480 ~ 660 nm), which suggest that it could be promisingly applied
in miniature mercury-free fluorescent lamps.
References
[1] RHODES, W H. Agglomerate and Particle Size Effects on Sintering Yttria-Stabilized Zirconia [J]. 1981, 64 (1):
19-22.
[2] Trave A, Buda F, Fasolino A. Band-Gap Engineering by III-V Infill in Sodalite [J]. Phys. Rev. Lett., 1996, 77
(27): 5405-5408.
[3] Wang Z, Wang Y, Liu B. Low Dimensional Effects on Luminescent Properties of CaWO4:Tb Nanophosphor
[J]. J. Electrochem. Soc., 2010, 157 (4): J125-J129.
[4] Igarashi T, Ihara M, Kusunoki T, Ohno K, Isobe T, Senna M.Relationship between optical properties and
crystallinity of nanometer Y2O3:Eu phosphor [J]. Appl. Phys. Lett., 2000, 76 (12): 1549-1551.
[5] Hou Z, Chai R, Zhang M, Zhang C, Chong P, Xu Z, Li G, Lin J. Fabrication and Luminescence Properties of
One-Dimensional CaMoO4: Ln3+ (Ln = Eu, Tb, Dy) Nanofibers via Electrospinning Process [J]. Langmuir, 2009,
25 (20): 12340-12348.
[6] Jüstel T, Nikol H, Ronda C. New Developments in the Field of Luminescent Materials for Lighting and
Displays [J]. Angew. Chem. Int. Ed., 1998, 37 (22): 3084-3103.
[7] Liu S, Zhao G, Lin X, Ying H, Liu J, Wang J, Han G. White luminescence of Tm-Dy ions co-doped
aluminoborosilicate glasses under UV light excitation [J]. J. Solid State Chem., 2008, 181 (10): 2725-2730.
[8] Su Q, Liang H, Li C, He H, Lu Y, Li J, Tao Y. Luminescent materials and spectroscopic properties of Dy3+
ion [J]. J. Lumin., 2007, 122-123: 927-930.
[9] Han G, Wang Y, Wu C, Zhang J. Hydrothermal synthesis and vacuum ultraviolet-excited luminescence
properties of novel Dy3+-doped LaPO4 white light phosphors [J]. Mater. Res. Bull., 2009, 44 (12):2255-2257.
[10] Bao A, Yang H, Tao C, Zhang Y, Han L. Luminescent properties of nanoparticles YPXV1−XO4:Dy
phosphors [J]. J. Lumin., 2008, 128 (1):60-66.
[11] Gou J, Wang Y. Adjustable Full-Color-Emitting Dy3+-Activated SrB4O7 by Codoping Zn2+ [J].
Electrochem. Solid-State Lett., 2007, 10 (8): F31-F33.
[12] Li L, Su Y, Li G. Size-induced symmetric enhancement and its relevance to photoluminescence of scheelite
CaWO4 nanocrystals [J]. Appl. Phys. Lett., 2007, 90: 054105-054107.
[13] Belsky A, Klimov S, Mikhailin V, Vasil'ev A, Auffray E, Lecoq P, Pedrini C, Korzhik M, Annenkov A,
Chevallier P, Chem. Phys. Lett., Influence of stoichiometry on the optical properties of lead tungstate crystals [J].
1997, 277 (1-3): 65-70.
[14] Gao X, Wang Y, Wang D, Liu B. Luminescent properties of KGd1−x(WO4)2:Eux3+ and
KGd1−x(WO4)2−y(MoO4)y:Eux3+ phosphors in UV-VUV regions [J]. J. Lumin., 129 (8): 840-843.
[15] Su Y, Li L, Li G. Generation of tunable wavelength lights in core-shell CaWO4 microspheres via co-doping
with Na+ and Ln3+ (Ln = Tb, Sm, Dy, Eu) [J]. J. Mater. Chem., 2009, 19:2316-2322.
[16] Wang H, Ma X, Qian X, Yin J, Zhu Z. Selective synthesis of CdWO4 short nanorods and nanofibers and their
self-assembly [J]. J. Solid State Chem., 177 (12): 4588-4596.
[17] Kim N, Choi S, Lee H, Kim K. Nanostructures and luminescence properties of porous ZnO thin films
prepared by sol-gel process [J]. Curr. Appl. Phys., 2009, 9 (3): 643-646.
[18] Dorenbos P. The 5d level positions of the trivalent lanthanides in inorganic compounds [J]. J. Lumin., 2000,
91 (3-4): 155-176.
[19] Aruna V, Hussain N, Buddhudu S. Spectra of Sm3 and Dy3: B2O3-P2O5-R2SO4 Glasses [J]. Mater. Res.
Bull., 1998, 33 (1): 149-159.
[20] Dominiak-Dzik G, Ryba-Romanowski W, Kovács L, Beregi E. Effect of temperature on luminescence and
VUV to visible conversion in the YAl3(BO3)4: Dy3+ (YAB: Dy) crystal [J]. Radiat. Meas. 2004, 38: 557.
- 7 -
�
215
220
225
中国科技论文在线
http://www.paper.edu.cn
CaWO4:Dy3+,Na+纳米球形白色发光材料在真空
紫外下发光性能的研究
(兰州大学物理科学与技术学院材料科学系,兰州 730000)
王赵锋,王育华
摘要:纳米发光材料以其独特的发光性能以及广阔的应用前景,受到了越来越多研究者的关
注。本论文采用水热法成功地制备了具有球形形貌、粒径为 70nm 左右的真空紫外(VUV)白
色发光材料 CaWO4:Dy3+,Na+,并对其 VUV 发光性能进行了研究。CaWO4:Dy3+,Na+纳米发光
材料的激发光谱表明,其在 VUV 区域存在很强的吸收,同时也反映了 CaWO4 基质可以有效地
将吸收来的能量传递给 Dy3+。当在 147nm 激发下,除了 Dy3+特征的偏黄色的 f-f 跃迁发射,
CaWO4:Dy3+,Na+纳米发光材料还表现出了钨酸根离子典型的蓝色发射,这使得该纳米发光材
料具有非常好的白光色纯度。此外,在激发和发射光谱分析的基础上,该纳米材料白色发光
的过程也得到了分析。综合发射强度以及色纯度,最佳纳米发光材料样品确定为 CaWO4:
0.01Dy3+,0.01Na+,其在微型发光设备中具有很大的应用潜力。
关键词:光致发光;纳米发光材料;真空紫外
中图分类号:O611.3
- 8 -
�












 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc