U N D E R S E A R O B O T S
Ultragentle manipulation of delicate structures using
a soft robotic gripper
Nina R. Sinatra1*, Clark B. Teeple1, Daniel M. Vogt1, Kevin Kit Parker1,
David F. Gruber2,3, Robert J. Wood1
Copyright © 2019
The Authors, some
rights reserved;
exclusive licensee
American Association
for the Advancement
of Science. No claim
to original U.S.
Government Works
Here, we present ultragentle soft robotic actuators capable of grasping delicate specimens of gelatinous marine
life. Although state-of-the-art soft robotic manipulators have demonstrated gentle gripping of brittle animals
(e.g., corals) and echinoderms (e.g., sea cucumbers) in the deep sea, they are unable to nondestructively grasp
more fragile soft-bodied organisms, such as jellyfish. Through an exploration of design parameters and laboratory
testing of individual actuators, we confirmed that our nanofiber-reinforced soft actuators apply sufficiently low
contact pressure to ensure minimal harm to typical jellyfish species. We then built a gripping device using several
actuators and evaluated its underwater grasping performance in the laboratory. By assessing the gripper’s region
of acquisition and robustness to external forces, we gained insight into the necessary precision and speed with
which grasping maneuvers must be performed to achieve successful collection of samples. Last, we demonstrated
successful manipulation of three live jellyfish species in an aquarium setting using a hand-held prototype gripper.
Overall, our ultragentle gripper demonstrates an improvement in gentle sample collection compared with existing
deep-sea sampling devices. Extensions of this technology may improve a variety of in situ characterization techniques
used to study the ecological and genetic features of deep-sea organisms.
INTRODUCTION
The marine environment has long been a deep well of bioinspiration for
previously unexplored materials and structures (1–3), cutting-edge medical
treatments (4, 5), and biomimetic manipulators and locomotors (6–11).
However, it is less common for these inventions to have practical
applications toward the biology and ecology of the animals from which
they are inspired. Gelatinous macroplankton—including cnidarian
medusae, ctenophores, and pelagic tunicates—are becoming increasingly
recognized as key ecosystem consumers of energy and nutrients
(12, 13) and are estimated to constitute a global biomass of 38.3 Tg C
(14). For many years, gelatinous zooplankton were overlooked by
marine scientists, largely because of a lack of delicate equipment to
study them, and have been referred to as “forgotten fauna” (15).
Discoveries such as life cycle reversal (16) and green fluorescent
protein (17, 18) are two examples of findings from gelatinous
zooplankton that have cross-disciplinary impacts and demonstrate
the importance of learning more about these life-forms.
Despite this vast potential, collecting intact samples of gelatinous
organisms to study remains extremely challenging. For example,
jellyfish are composed of more than 95% water, and their mesogleal
tissue has an extremely low stiffness (Young’s modulus of 0.34 to
1.2 kPa) (19–22). Capturing these delicate creatures in the ocean
has been a challenge for the research community because existing
technologies (e.g., nets and vacuum devices) frequently damage
samples during the collection process (15, 23). The ideal grasping
device for delicate materials in dynamic domains must incorporate
a soft and flexible interface, compliant yet tough (able to absorb
energy and deform plastically before fracture) appendages, and a
radius of curvature compatible with the target size. This work focuses
1John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute
for Biologically Inspired Engineering, Harvard University, 29 Oxford Street, Cambridge,
MA 02138, USA. 2Department of Natural Sciences, Baruch College, City University
of New York, 55 Lexington Ave., New York, NY 10010, USA. 3PhD Program in Biology,
Graduate Center, City University of New York, 365 5th Ave., New York, NY 10016, USA.
*Corresponding author. Email: sinatra.nina@gmail.com
on developing a soft gripper with such properties. The target
organisms for this device are three small (roughly 7 to 10 cm) jellyfish
species: Aurelia aurita, Catostylus mosaicus, and Mastigias papua.
Jellyfish present extreme challenges for delicate grasping and
manipulation, and lessons learned from this exploration may be
transferred to other tasks involving fragile or gelatinous objects.
Currently, state-of-the-art aquatic grippers can be classified into
five categories: (i) forked metal or plastic jaws (24, 25), (ii) soft
hydraulic actuators (26, 27), (iii) jamming grippers (28), (iv) suction
samplers (29), and (v) noncontact containers that close around a
swimming animal (30). Metal or plastic grippers are the most widespread
marine sampling method due to their use in oil and natural gas
industries, but their rigid surfaces can snag or compress gelatinous
animals, and a fixed radius of curvature is unable to conform
controllably to the shape of amorphous or soft-bodied animals (31).
Foam-coated hydraulic actuators have substantially improved non-
destructive sampling of delicate benthic and midwater animals (e.g.,
coral and sea stars) by decreasing grip force while increasing pressure
distribution (26). However, the contact pressure exerted by foam-covered
actuators (1 to 10 kPa) is too high to grasp gelatinous animals
without causing harm (fig. S4). Although particle jamming grippers
are capable of lifting sedentary objects, their operating mechanism
is not well suited for grasping floating organisms (28, 32). Suction
samplers use a pump to draw an untethered animal through an inlet
tube and into a storage container; however, delicate organisms can
be damaged as they move through the tubing (30, 33).
Last, noncontact tools such as the detritus sampler (“D-Sampler”)
or the RAD (rotary-actuated dodecahedron) sampler are open
containers that are positioned near floating specimens and then closed
around the organism. Use of the D-Sampler with remotely operated
vehicles (ROVs) is challenging because the container is typically
positioned by moving the entire vehicle (30, 34). Although the RAD
sampler (30) has successfully captured centimeter-scale squid and octopi,
misalignment of the container (an origami-inspired structure that
folds to form a sealed dodecahedron) as it closes can trap and cleave
1 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
the tentacles of fragile specimens. A flexible, compliant gripper is needed
to bridge this capability gap, thereby achieving nondestructive grasping
of gelatinous marine organisms. In this study, we report a multi-scale
approach for gentle grasping using nanofiber-reinforced soft actuators.
Previously, we developed flexible and robust microscale soft
actuators composed of an elastomer matrix reinforced with polymer
nanofibers (35). Nanofibers were selected as the strain-limiting layer
because of their ease of processing and high specific strength; the
actuators underwent large deformations without requiring stiffer
reinforcing materials, such as carbon or glass fibers. Furthermore,
the nanofabric’s high surface area facilitated bonding with elastomeric
materials during the fabrication processes and demonstrated no
delamination from the matrix after tensile testing of the actuator to
failure. Unlike our previous nanofiber-reinforced polydimethylsiloxane
microrobots (whose matrix has a Shore hardness of 43), the mesoscale
actuators presented here incorporate a lower durometer silicone matrix
(Shore 20A), which is better suited for interacting with fragile structures.
Here, we leverage the flexibility and durability of these materials to
engineer mesoscale soft hydraulic actuators.
Our grasping device is composed of six composite actuator “fingers”
connected to a three-dimensional (3D)–printed “palm” produced using
a PolyJet-based printer (Objet Connex500, Stratasys). Each actuator
contains an elastic yet tough silicone matrix and a strain-limiting
layer of flexible polymer nanofibers. The gripper is lightweight (123 g)
and can be actuated using very low hydraulic pressure (1 to 6 psi,
or 6.9 to 41.4 kPa, with respect to ambient).
Upon pressurization, a channel within each actuator inflates, and
the appendage bends in the direction of the stiffer fiber-reinforced
layer. Inflation of several actuators spaced around a marine organism
enables the animal to be gently cradled by the soft silicone digits.
The actuators overlap and contact one another, forming a soft
network that restricts the position of the target but does not fully
immobilize it (36); this caging grasp reduces the need to precisely
control individual finger placement and instead relies on collective
inflation of all actuators. The low contact pressure exerted by each
actuator (0.0455 ± 0.007 kPa) also facilitates nondestructive interaction.
To better understand the device’s performance space, we empirically
identified the region of acquisition using a synthetic target and
quantified the resistance to forces on the object during a grasp. The
soft gripper can be adapted both to deep-sea exploration using an
ROV (Fig. 1A) and to portable specimen collection in the shallower
waters of the photic zone. Last, we demonstrate the use of the portable
device setup to successfully grasp three jellyfish species (Fig. 1, B to D).
RESULTS
Design criteria for ultragentle gripper
The objective for this device is to perform nondestructive grasping
of gelatinous marine organisms in the marine environment. We
identified several performance requirements to ensure successful
function. First, because the gripper will be fully submerged in the
ocean during operation, it must be composed of materials that are
resistant to corrosion in salt water. To fulfill this aim, we selected a
silicone matrix, nylon and polyurethane reinforcing fiber, PolyJet
palm chassis, and stainless steel fasteners, each of which is undamaged
by seawater immersion during the operational life cycle of this
device (several weeks) (37–41). Second, the materials must withstand
use at typical ocean temperatures, which reach a minimum of 0° to
3°C in the deep sea (several thousand meters) and a surface average
of 17°C. The operational range of all the materials listed above is
within this window; for example, silicone rubbers are highly resistant
to low temperatures and have an embrittlement temperature between
−20° and −30°C (42). Because of the design of the hydraulic pump
used to inflate the soft fingers (26), pressure within the actuators is
equalized to that of the surrounding waters and is therefore not a
barrier to operation.
The following design criteria relate to the organisms that the
gripper will be required to interact with. The actuator length must
be determined by the average size of the intended object or species.
Although gelatinous animals exist in a variety of shapes and sizes,
we selected three of the most widely studied jellyfish species to serve
as models: A. aurita, C. mosaicus, and M. papua. The average bell
diameter for these species is 7 to 10 cm (43, 44). To fully enclose the
body of the selected jellyfish, the total length for our actuators must
exceed this amount and was set at 15 cm. However, actuator size
may be easily scaled to accommodate larger or smaller organisms.
Larger actuators may require thicker nanofiber sheets near the distal
tip to maintain uniform curvature throughout the length of the
actuator. During extended operation, a small amount of air can
become trapped in this area of the internal channel. The silicone
membrane will deform more around the enclosed air than around
water, leading to higher local curvature at the distal end (i.e., the
“finger tip” will bend more than the rest of the actuator). Last, to
execute a nondestructive grasp, the contact pressure exerted by each
actuator should be below 1 kPa, the current state of the art for soft
marine grippers (26). We have evaluated the pressure exerted by
our composite actuators in a subsequent section.
Robotic palm design
The soft robotic gripper includes six modular actuators attached to
a custom-designed, 3D-printed hub (Fig. 2B) composed of a transparent
photopolymer (RGD720, Stratasys). The appendages are individually
attached to the central palm and may be removed or exchanged in
the case of actuator failure or to experiment with different digit
configurations. The set of actuators are pressurized hydraulically
(Fig. 2C) using a single channel at the back of the hub, which can be
attached to a fluid source using quick-disconnect fittings. In the
present configuration, the actuators were evenly spaced, with two
on either side of a 78-mm rectangular palm and one on either end
of the opposite 45-mm edge. This layout was chosen because adhesion
and friction between actuators on either side of the hub resulted in
a strong caging grasp (fig. S7). The actuators placed at the top and
bottom of the palm prevent a target object from being released at
either end and contribute to a caging grasp by overlapping the center
actuators. Actuator position can be rapidly adjusted by modifying
the 3D-printed hub design. Additive manufacturing supports iterative
development of soft grippers with varying numbers and positions of
actuators (e.g., a hexagonal hub with six actuators or a circular palm
with 10 digits). This flexibility can enable further customization to
support organisms with specific shapes and symmetries (27).
Actuator design
Understanding the impact of geometric and process variables during
actuator fabrication is crucial to regulating input pressure range
and reducing manufacturing defects. Three key design parameters
can be tuned toward achieving these goals: interior channel height,
thickness of the inflating membrane, and thickness of the adhesive
layer during the cobonding process (illustrated in Fig. 3A). Each of
2 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
A
Delicate Gripping
< 1 kPa
< 1 kPa
< 1 kPa
B
C
D
2 cm
2 cm
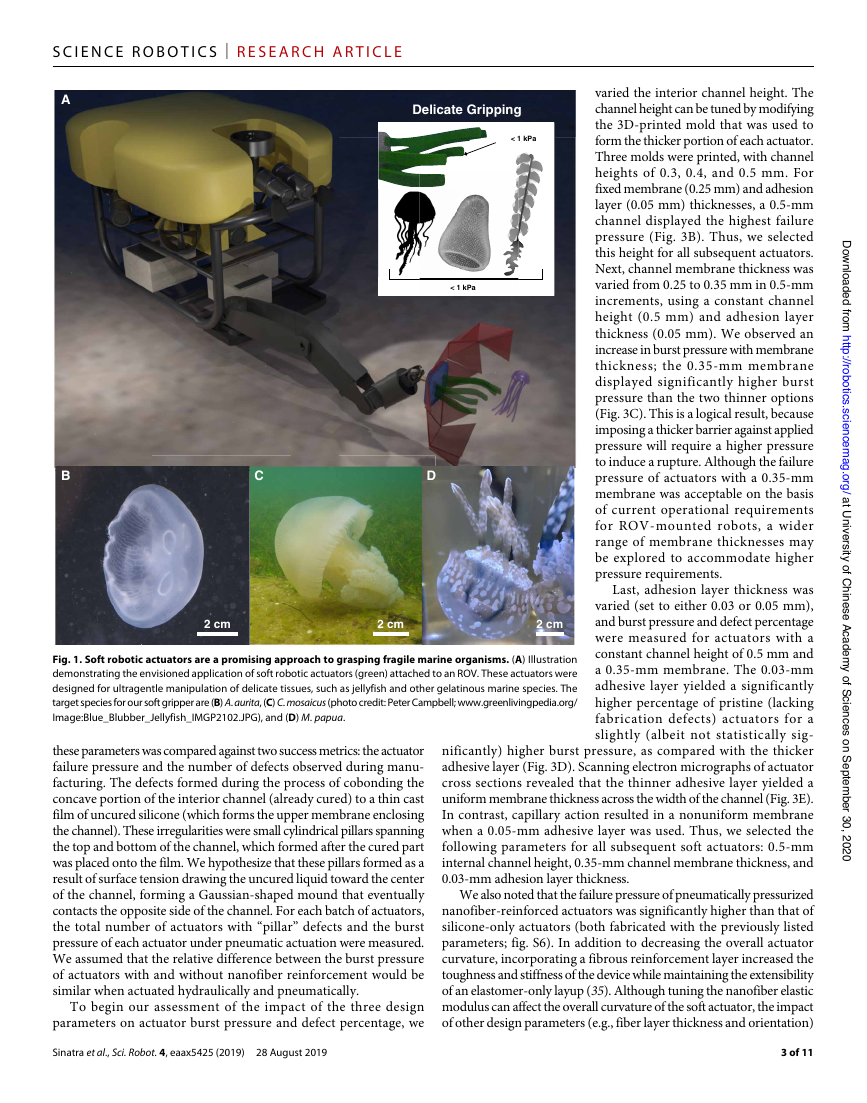
Fig. 1. Soft robotic actuators are a promising approach to grasping fragile marine organisms. (A) Illustration
demonstrating the envisioned application of soft robotic actuators (green) attached to an ROV. These actuators were
designed for ultragentle manipulation of delicate tissues, such as jellyfish and other gelatinous marine species. The
target species for our soft gripper are (B) A. aurita, (C) C. mosaicus (photo credit: Peter Campbell; www.greenlivingpedia.org/
Image:Blue_Blubber_Jellyfish_IMGP2102.JPG), and (D) M. papua.
these parameters was compared against two success metrics: the actuator
failure pressure and the number of defects observed during manu-
facturing. The defects formed during the process of cobonding the
concave portion of the interior channel (already cured) to a thin cast
film of uncured silicone (which forms the upper membrane enclosing
the channel). These irregularities were small cylindrical pillars spanning
the top and bottom of the channel, which formed after the cured part
was placed onto the film. We hypothesize that these pillars formed as a
result of surface tension drawing the uncured liquid toward the center
of the channel, forming a Gaussian- shaped mound that eventually
contacts the opposite side of the channel. For each batch of actuators,
the total number of actuators with “pillar” defects and the burst
pressure of each actuator under pneumatic actuation were measured.
We assumed that the relative difference between the burst pressure
of actuators with and without nanofiber reinforcement would be
similar when actuated hydraulically and pneumatically.
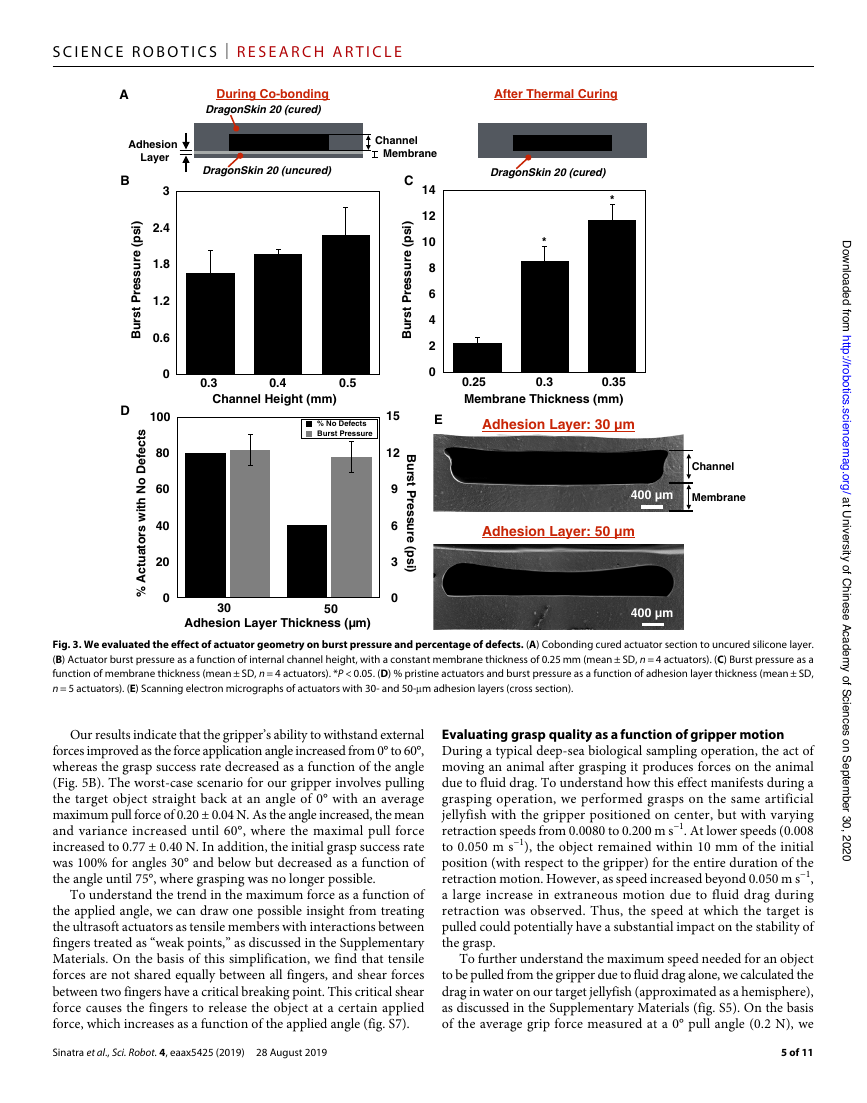
To begin our assessment of the impact of the three design
parameters on actuator burst pressure and defect percentage, we
varied the interior channel height. The
channel height can be tuned by modifying
the 3D-printed mold that was used to
form the thicker portion of each actuator.
Three molds were printed, with channel
heights of 0.3, 0.4, and 0.5 mm. For
fixed membrane (0.25 mm) and adhesion
layer (0.05 mm) thicknesses, a 0.5-mm
channel displayed the highest failure
pressure (Fig. 3B). Thus, we selected
this height for all subsequent actuators.
Next, channel membrane thickness was
varied from 0.25 to 0.35 mm in 0.5-mm
increments, using a constant channel
height (0.5 mm) and adhesion layer
thickness (0.05 mm). We observed an
increase in burst pressure with membrane
thickness; the 0.35-mm membrane
displayed significantly higher burst
pressure than the two thinner options
(Fig. 3C). This is a logical result, because
imposing a thicker barrier against applied
pressure will require a higher pressure
to induce a rupture. Although the failure
pressure of actuators with a 0.35-mm
membrane was acceptable on the basis
of current operational requirements
for ROV-mounted robots, a wider
range of membrane thicknesses may
be explored to accommodate higher
pressure requirements.
2 cm
Last, adhesion layer thickness was
varied (set to either 0.03 or 0.05 mm),
and burst pressure and defect percentage
were measured for actuators with a
constant channel height of 0.5 mm and
a 0.35-mm membrane. The 0.03-mm
adhesive layer yielded a significantly
higher percentage of pristine (lacking
fabrication defects) actuators for a
slightly (albeit not statistically sig-
nificantly) higher burst pressure, as compared with the thicker
adhesive layer (Fig. 3D). Scanning electron micrographs of actuator
cross sections revealed that the thinner adhesive layer yielded a
uniform membrane thickness across the width of the channel (Fig. 3E).
In contrast, capillary action resulted in a nonuniform membrane
when a 0.05-mm adhesive layer was used. Thus, we selected the
following parameters for all subsequent soft actuators: 0.5-mm
internal channel height, 0.35-mm channel membrane thickness, and
0.03-mm adhesion layer thickness.
We also noted that the failure pressure of pneumatically pressurized
nanofiber-reinforced actuators was significantly higher than that of
silicone-only actuators (both fabricated with the previously listed
parameters; fig. S6). In addition to decreasing the overall actuator
curvature, incorporating a fibrous reinforcement layer increased the
toughness and stiffness of the device while maintaining the extensibility
of an elastomer-only layup (35). Although tuning the nanofiber elastic
modulus can affect the overall curvature of the soft actuator, the impact
of other design parameters (e.g., fiber layer thickness and orientation)
3 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
A
1
Mold Air Channel
Nanofiber Sheet
B
C
2
3
4
5
6
Thermally Cure,
Remove from Mold
Prepare Membrane
with Film Applicator
Place Cured Part on
Uncured Membrane
Thermally Cure,
Remove from Film
Membrane
Insert Tubing, Seal
Epoxy
KEY:
Uncured silicone
Cured silicone
Film applicator surface
3D printed mold
1 cm
Fig. 2. Fabricating nanofiber-reinforced soft robotic actuators. (A) Actuators were manufactured using molding
and cobonding (cross-sectional view shown). (B) Six soft actuators connected to a 3D-printed hub. (C) As an actuator
is pressurized, the internal channel inflates and the device bends toward the strain-limiting nanofiber layer.
Nanofiber Sheet
Fully Pressurized
jellyfish and because its bell diameter is
on the order of that of the species of
interest for this study. To capture the
entire region, we performed a sweep
over a grid of centering positions (n = 2
grasps per position) 160 mm wide and
120 mm high, with a step size of 10 mm
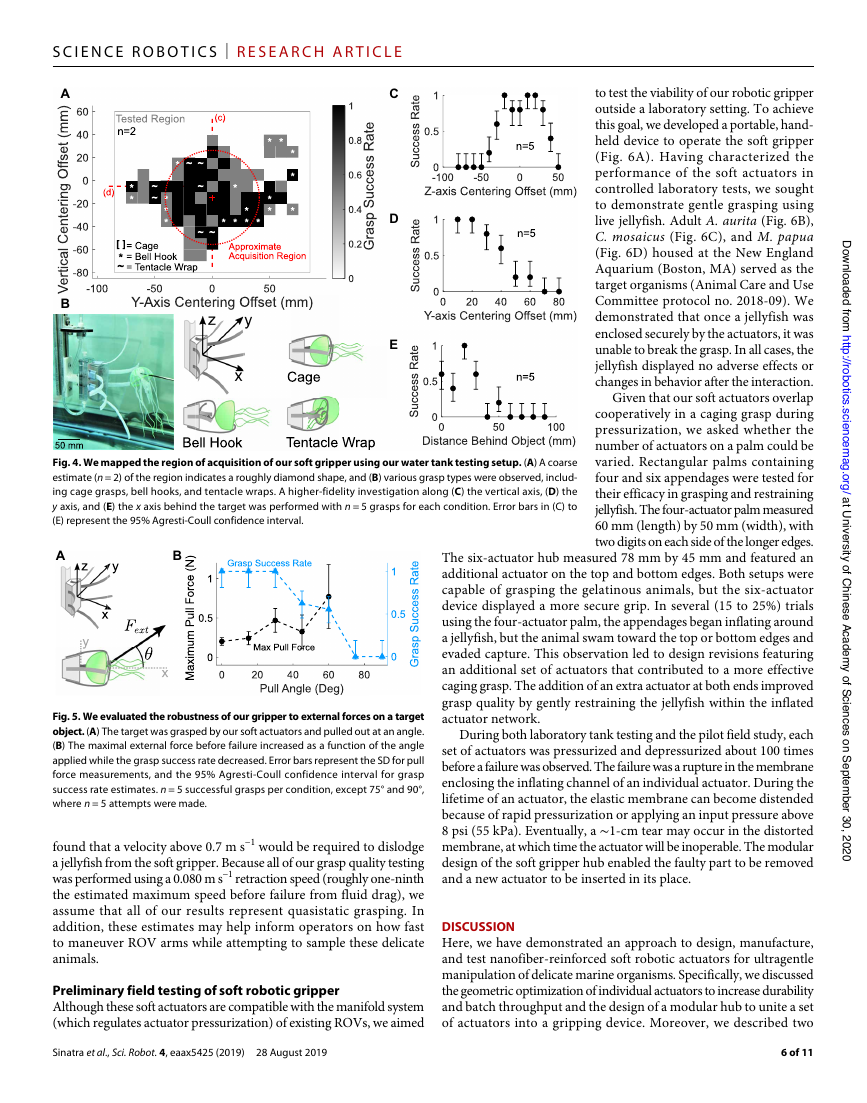
in both directions (Fig. 4A).
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Channel
At Rest
Partially Pressurized
To quantitatively asses gripper perform-
ance at each centering position, we
determined the grasp success rate by
observing the number of successful grasps.
A grasp was considered successful if the
target was moved to its final position and
remained there until it was released. If
the target was released early or was not
grasped, the attempt was considered a
failure. The grasp success rate at a given
hand position was then calculated as the
number of successful grasps normalized
by the total number of attempts. Further
refinement of the acquisition region was obtained by performing
higher-repetition sweeps (n = 5) along key axes associated with the
3D volume of the region. The axes tested include the vertical axis (z)
through the center of the region, one side of the horizontal axis (y)
through the center, and the axis directly behind the center of the
object (x). The resulting grasp success rates are shown in Fig. 4 (C to E).
By examining the shape of the region where successful grasps
occur, we can quantify the positioning and precision necessary for
reliable grasping. The general shape of the region is a diamond
(roughly symmetric about the vertical axis), as shown in Fig. 4A.
This shape is logical considering the placement of actuators around
the rectangular palm. In addition, unsurprisingly, the center of the
main region is located 10 mm below the center of the object, which
can be explained by slight buoyancy of the soft actuators due to
trapped air. The characteristic dimension of the region of acquisition
can be found by the radius of the largest circle that can be completely
contained within the boundaries of the main region (conservative
metric). For our gripper, this radius is about 42 mm. Last, we
observed that grasps toward the center of the region consistently
demonstrated caging grasps, whereas locations along the edge of
the region displayed a wider variety of grasps (hooking under the
bell or wrapping around tentacles).
Gripper’s robustness to external forces
The robustness to external forces and torques on an object during a
grasp is typically defined as the maximum force the gripper can
resist (or conversely, the minimum force required to pull an object
out of the grasp), minimized over all force application angles (46).
This measure of robustness can be used to understand how to
maneuver an ROV arm to transport delicate samples to a storage
container. To quantify how the angle of applied force affects the
robustness, we executed grasps on the same synthetic jellyfish
target. After grasping, force was applied at a prescribed angle until
the target slipped out of the gripper. The process was repeated five
times for angles ranging from 0° (perpendicular to the palm) to 90°
(parallel to the palm) in increments of 15° (Fig. 5A). We assumed
that the relationship is symmetric about the x-z plane based on the
symmetry of actuator placement.
4 of 11
may be investigated in future studies using analytical models developed
in a previous analysis (35).
Quantifying contact pressure exerted by
individual actuators
One benefit of using soft robotic actuators for gentle manipulation
is that the contact pressure exerted by each appendage can be lower
than that of traditional rigid (e.g., metal) end effectors. To assess the
contact pressure of our device, we pressurized individual actuators
while in contact with a stationary load cell. At their typical operating
pressure of 6 psi (41.4 kPa), nanofiber-reinforced soft actuators
exerted an average contact pressure of 0.0455 ± 0.007 kPa
(mean ± SEM), which is well below the target of <1 kPa. A representative
plot of one actuator being hydraulically pressurized and depressurized
over four cycles in shown in fig. S2B. These results confirm that our soft
actuators exert a low, safe pressure on target objects and organisms.
Evaluation of underwater grasping performance
The performance of our ultrasoft robotic gripper was empirically
evaluated on the basis of two standard grasp quality metrics: the size
of the region of acquisition (45) and the robustness to external forces
on the object (46, 47). Both metrics can be used to inform potential
end users (ROV pilots) about how to best use the capabilities of this
gripper when mounted on a teleoperated ROV.
Gripper’s region of acquisition
The region of acquisition of a grasping device is defined as the set of
all positions (relative to a target object) that the gripper can be
placed to reliably grasp the target (45). For this ultragentle hand, we
first made a rough map of a planar slice of this 3D region and then
performed higher-fidelity testing along key axes. On the basis of the
estimated region of acquisition, we can gain insight into the precision
required to reliably grasp objects when the hand is mounted to the
arm of an ROV.
A slice of the region of acquisition (located about 5 mm behind
the target) was estimated empirically by performing a series of
grasps on a surrogate target: a silicone model jellyfish. This target
was selected on the basis of its morphological similarity to a living
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
A
During Co-bonding
DragonSkin 20 (cured)
After Thermal Curing
Adhesion
Layer
B
)
i
s
p
(
e
r
u
s
s
e
r
P
t
s
r
u
B
3
2.4
1.8
1.2
0.6
0
D
100
s
t
c
e
f
e
D
o
N
h
t
i
w
s
r
o
t
a
u
t
c
A
%
DragonSkin 20 (uncured)
0.3
0.4
0.5
Channel Height (mm)
% No Defects
Burst Pressure
80
60
40
20
0
30
Adhesion Layer Thickness (µm)
50
Channel
Membrane
C
)
i
s
p
(
e
r
u
s
s
e
r
P
t
s
r
u
B
14
12
10
8
6
4
2
0
DragonSkin 20 (cured)
*
*
0.25
0.35
Membrane Thickness (mm)
0.3
E
Adhesion Layer: 30 µm
Channel
400 µm
Membrane
Adhesion Layer: 50 µm
15
12
9
6
3
0
B
u
r
s
t
P
r
e
s
s
u
r
e
(
p
s
i
)
400 µm
Fig. 3. We evaluated the effect of actuator geometry on burst pressure and percentage of defects. (A) Cobonding cured actuator section to uncured silicone layer.
(B) Actuator burst pressure as a function of internal channel height, with a constant membrane thickness of 0.25 mm (mean ± SD, n = 4 actuators). (C) Burst pressure as a
function of membrane thickness (mean ± SD, n = 4 actuators). *P < 0.05. (D) % pristine actuators and burst pressure as a function of adhesion layer thickness (mean ± SD,
n = 5 actuators). (E) Scanning electron micrographs of actuators with 30- and 50-m adhesion layers (cross section).
Our results indicate that the gripper’s ability to withstand external
forces improved as the force application angle increased from 0° to 60°,
whereas the grasp success rate decreased as a function of the angle
(Fig. 5B). The worst-case scenario for our gripper involves pulling
the target object straight back at an angle of 0° with an average
maximum pull force of 0.20 ± 0.04 N. As the angle increased, the mean
and variance increased until 60°, where the maximal pull force
increased to 0.77 ± 0.40 N. In addition, the initial grasp success rate
was 100% for angles 30° and below but decreased as a function of
the angle until 75°, where grasping was no longer possible.
To understand the trend in the maximum force as a function of
the applied angle, we can draw one possible insight from treating
the ultrasoft actuators as tensile members with interactions between
fingers treated as “weak points,” as discussed in the Supplementary
Materials. On the basis of this simplification, we find that tensile
forces are not shared equally between all fingers, and shear forces
between two fingers have a critical breaking point. This critical shear
force causes the fingers to release the object at a certain applied
force, which increases as a function of the applied angle (fig. S7).
Evaluating grasp quality as a function of gripper motion
During a typical deep-sea biological sampling operation, the act of
moving an animal after grasping it produces forces on the animal
due to fluid drag. To understand how this effect manifests during a
grasping operation, we performed grasps on the same artificial
jellyfish with the gripper positioned on center, but with varying
retraction speeds from 0.0080 to 0.200 m s−1. At lower speeds (0.008
to 0.050 m s−1), the object remained within 10 mm of the initial
position (with respect to the gripper) for the entire duration of the
retraction motion. However, as speed increased beyond 0.050 m s−1,
a large increase in extraneous motion due to fluid drag during
retraction was observed. Thus, the speed at which the target is
pulled could potentially have a substantial impact on the stability of
the grasp.
To further understand the maximum speed needed for an object
to be pulled from the gripper due to fluid drag alone, we calculated the
drag in water on our target jellyfish (approximated as a hemisphere),
as discussed in the Supplementary Materials (fig. S5). On the basis
of the average grip force measured at a 0° pull angle (0.2 N), we
5 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
Fig. 4. We mapped the region of acquisition of our soft gripper using our water tank testing setup. (A) A coarse
estimate (n = 2) of the region indicates a roughly diamond shape, and (B) various grasp types were observed, includ-
ing cage grasps, bell hooks, and tentacle wraps. A higher-fidelity investigation along (C) the vertical axis, (D) the
y axis, and (E) the x axis behind the target was performed with n = 5 grasps for each condition. Error bars in (C) to
(E) represent the 95% Agresti-Coull confidence interval.
A
B
C
D
E
A
B
Fig. 5. We evaluated the robustness of our gripper to external forces on a target
object. (A) The target was grasped by our soft actuators and pulled out at an angle.
(B) The maximal external force before failure increased as a function of the angle
applied while the grasp success rate decreased. Error bars represent the SD for pull
force measurements, and the 95% Agresti-Coull confidence interval for grasp
success rate estimates. n = 5 successful grasps per condition, except 75° and 90°,
where n = 5 attempts were made.
found that a velocity above 0.7 m s−1 would be required to dislodge
a jellyfish from the soft gripper. Because all of our grasp quality testing
was performed using a 0.080 m s−1 retraction speed (roughly one-ninth
the estimated maximum speed before failure from fluid drag), we
assume that all of our results represent quasistatic grasping. In
addition, these estimates may help inform operators on how fast
to maneuver ROV arms while attempting to sample these delicate
animals.
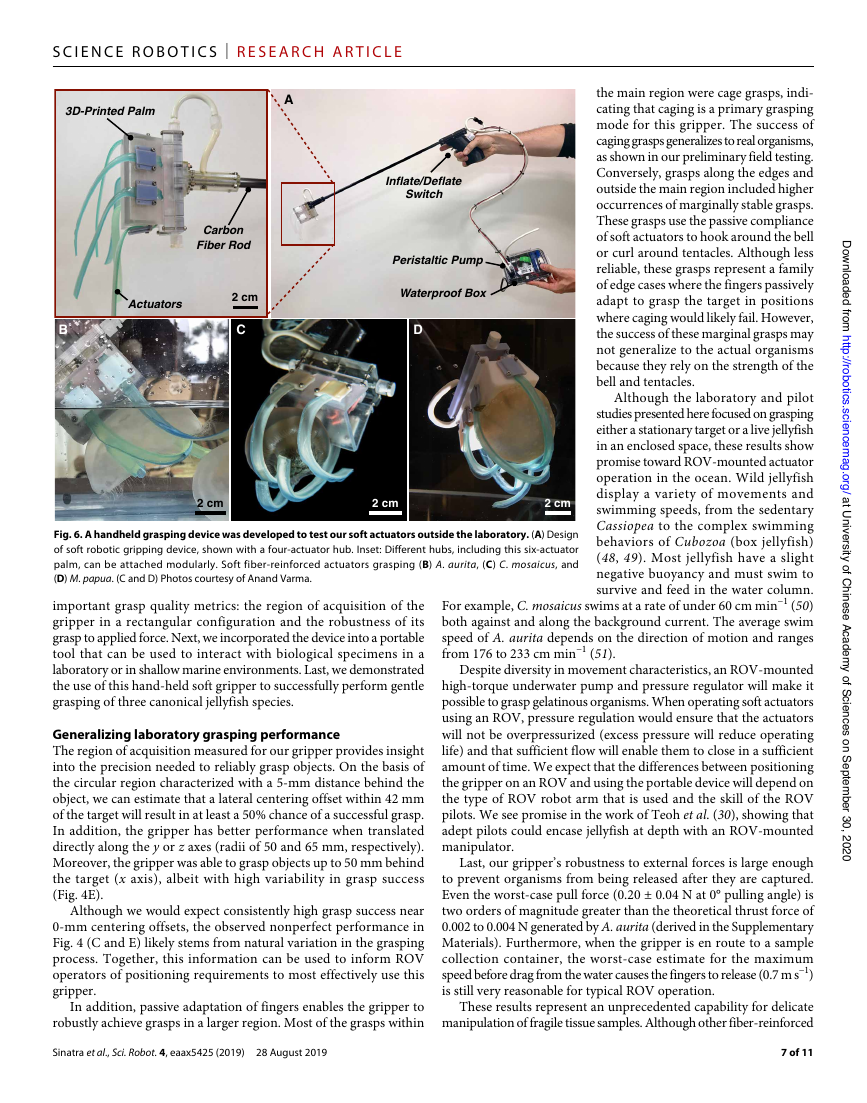
Preliminary field testing of soft robotic gripper
Although these soft actuators are compatible with the manifold system
(which regulates actuator pressurization) of existing ROVs, we aimed
to test the viability of our robotic gripper
outside a laboratory setting. To achieve
this goal, we developed a portable, hand-
held device to operate the soft gripper
(Fig. 6A). Having characterized the
performance of the soft actuators in
controlled laboratory tests, we sought
to demonstrate gentle grasping using
live jellyfish. Adult A. aurita (Fig. 6B),
C. mosaicus (Fig. 6C), and M. papua
(Fig. 6D) housed at the New England
Aquarium (Boston, MA) served as the
target organisms (Animal Care and Use
Committee protocol no. 2018-09). We
demonstrated that once a jellyfish was
enclosed securely by the actuators, it was
unable to break the grasp. In all cases, the
jellyfish displayed no adverse effects or
changes in behavior after the interaction.
Given that our soft actuators overlap
cooperatively in a caging grasp during
pressurization, we asked whether the
number of actuators on a palm could be
varied. Rectangular palms containing
four and six appendages were tested for
their efficacy in grasping and restraining
jellyfish. The four-actuator palm measured
60 mm (length) by 50 mm (width), with
two digits on each side of the longer edges.
The six-actuator hub measured 78 mm by 45 mm and featured an
additional actuator on the top and bottom edges. Both setups were
capable of grasping the gelatinous animals, but the six-actuator
device displayed a more secure grip. In several (15 to 25%) trials
using the four-actuator palm, the appendages began inflating around
a jellyfish, but the animal swam toward the top or bottom edges and
evaded capture. This observation led to design revisions featuring
an additional set of actuators that contributed to a more effective
caging grasp. The addition of an extra actuator at both ends improved
grasp quality by gently restraining the jellyfish within the inflated
actuator network.
During both laboratory tank testing and the pilot field study, each
set of actuators was pressurized and depressurized about 100 times
before a failure was observed. The failure was a rupture in the membrane
enclosing the inflating channel of an individual actuator. During the
lifetime of an actuator, the elastic membrane can become distended
because of rapid pressurization or applying an input pressure above
8 psi (55 kPa). Eventually, a ∼1-cm tear may occur in the distorted
membrane, at which time the actuator will be inoperable. The modular
design of the soft gripper hub enabled the faulty part to be removed
and a new actuator to be inserted in its place.
DISCUSSION
Here, we have demonstrated an approach to design, manufacture,
and test nanofiber-reinforced soft robotic actuators for ultragentle
manipulation of delicate marine organisms. Specifically, we discussed
the geometric optimization of individual actuators to increase durability
and batch throughput and the design of a modular hub to unite a set
of actuators into a gripping device. Moreover, we described two
6 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
3D-Printed Palm
A
Carbon
Fiber Rod
Actuators
B
2 cm
C
Inflate/Deflate
Switch
Peristaltic Pump
Waterproof Box
D
2 cm
2 cm
Fig. 6. A handheld grasping device was developed to test our soft actuators outside the laboratory. (A) Design
of soft robotic gripping device, shown with a four-actuator hub. Inset: Different hubs, including this six-actuator
palm, can be attached modularly. Soft fiber-reinforced actuators grasping (B) A. aurita, (C) C. mosaicus, and
(D) M. papua. (C and D) Photos courtesy of Anand Varma.
important grasp quality metrics: the region of acquisition of the
gripper in a rectangular configuration and the robustness of its
grasp to applied force. Next, we incorporated the device into a portable
tool that can be used to interact with biological specimens in a
laboratory or in shallow marine environments. Last, we demonstrated
the use of this hand-held soft gripper to successfully perform gentle
grasping of three canonical jellyfish species.
Generalizing laboratory grasping performance
The region of acquisition measured for our gripper provides insight
into the precision needed to reliably grasp objects. On the basis of
the circular region characterized with a 5-mm distance behind the
object, we can estimate that a lateral centering offset within 42 mm
of the target will result in at least a 50% chance of a successful grasp.
In addition, the gripper has better performance when translated
directly along the y or z axes (radii of 50 and 65 mm, respectively).
Moreover, the gripper was able to grasp objects up to 50 mm behind
the target (x axis), albeit with high variability in grasp success
(Fig. 4E).
Although we would expect consistently high grasp success near
0-mm centering offsets, the observed nonperfect performance in
Fig. 4 (C and E) likely stems from natural variation in the grasping
process. Together, this information can be used to inform ROV
operators of positioning requirements to most effectively use this
gripper.
In addition, passive adaptation of fingers enables the gripper to
robustly achieve grasps in a larger region. Most of the grasps within
the main region were cage grasps, indi-
cating that caging is a primary grasping
mode for this gripper. The success of
caging grasps generalizes to real organisms,
as shown in our preliminary field testing.
Conversely, grasps along the edges and
outside the main region included higher
occurrences of marginally stable grasps.
These grasps use the passive compliance
of soft actuators to hook around the bell
or curl around tentacles. Although less
reliable, these grasps represent a family
of edge cases where the fingers passively
adapt to grasp the target in positions
where caging would likely fail. However,
the success of these marginal grasps may
not generalize to the actual organisms
because they rely on the strength of the
bell and tentacles.
2 cm
Although the laboratory and pilot
studies presented here focused on grasping
either a stationary target or a live jellyfish
in an enclosed space, these results show
promise toward ROV-mounted actuator
operation in the ocean. Wild jellyfish
display a variety of movements and
swimming speeds, from the sedentary
Cassiopea to the complex swimming
behaviors of Cubozoa (box jellyfish)
(48, 49). Most jellyfish have a slight
negative buoyancy and must swim to
survive and feed in the water column.
For example, C. mosaicus swims at a rate of under 60 cm min−1 (50)
both against and along the background current. The average swim
speed of A. aurita depends on the direction of motion and ranges
from 176 to 233 cm min−1 (51).
Despite diversity in movement characteristics, an ROV-mounted
high-torque underwater pump and pressure regulator will make it
possible to grasp gelatinous organisms. When operating soft actuators
using an ROV, pressure regulation would ensure that the actuators
will not be overpressurized (excess pressure will reduce operating
life) and that sufficient flow will enable them to close in a sufficient
amount of time. We expect that the differences between positioning
the gripper on an ROV and using the portable device will depend on
the type of ROV robot arm that is used and the skill of the ROV
pilots. We see promise in the work of Teoh et al. (30), showing that
adept pilots could encase jellyfish at depth with an ROV-mounted
manipulator.
Last, our gripper’s robustness to external forces is large enough
to prevent organisms from being released after they are captured.
Even the worst-case pull force (0.20 ± 0.04 N at 0° pulling angle) is
two orders of magnitude greater than the theoretical thrust force of
0.002 to 0.004 N generated by A. aurita (derived in the Supplementary
Materials). Furthermore, when the gripper is en route to a sample
collection container, the worst-case estimate for the maximum
speed before drag from the water causes the fingers to release (0.7 m s−1)
is still very reasonable for typical ROV operation.
These results represent an unprecedented capability for delicate
manipulation of fragile tissue samples. Although other fiber-reinforced
7 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�
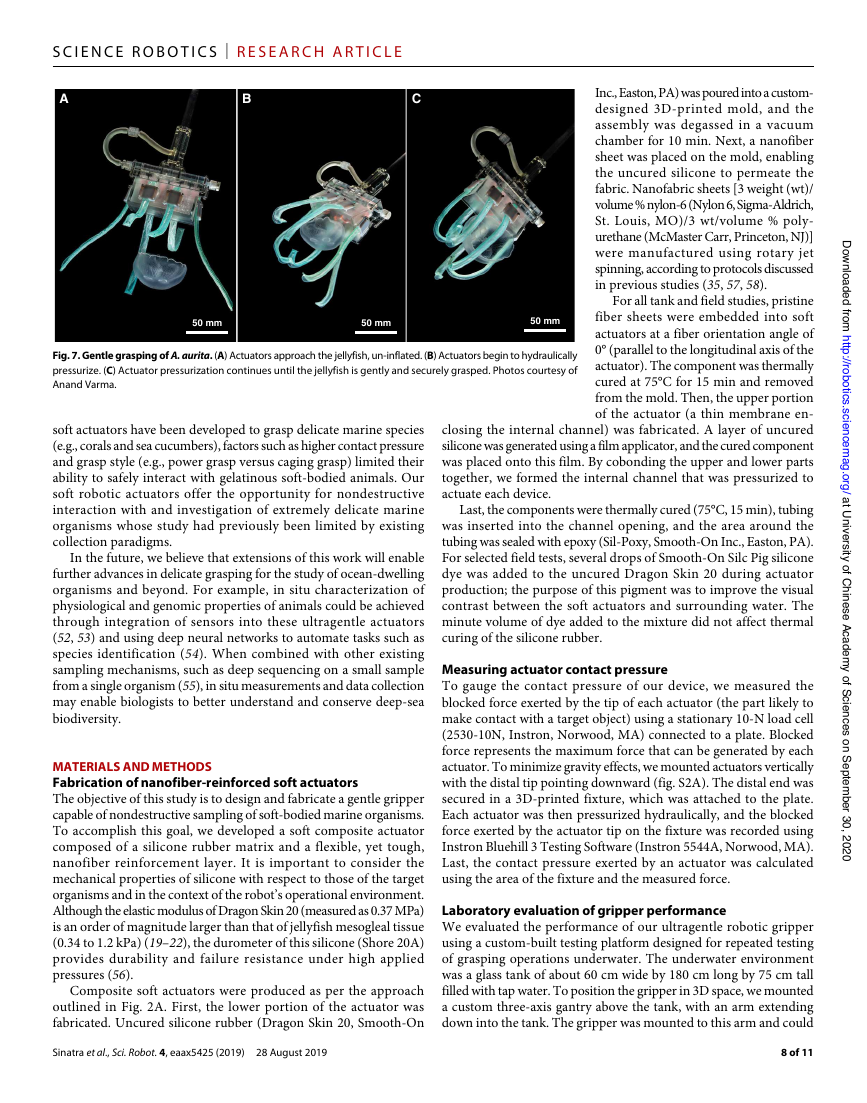
Fig. 7. Gentle grasping of A. aurita. (A) Actuators approach the jellyfish, un-inflated. (B) Actuators begin to hydraulically
pressurize. (C) Actuator pressurization continues until the jellyfish is gently and securely grasped. Photos courtesy of
Anand Varma.
A
B
C
50 mm
50 mm
soft actuators have been developed to grasp delicate marine species
(e.g., corals and sea cucumbers), factors such as higher contact pressure
and grasp style (e.g., power grasp versus caging grasp) limited their
ability to safely interact with gelatinous soft-bodied animals. Our
soft robotic actuators offer the opportunity for nondestructive
interaction with and investigation of extremely delicate marine
organisms whose study had previously been limited by existing
collection paradigms.
In the future, we believe that extensions of this work will enable
further advances in delicate grasping for the study of ocean-dwelling
organisms and beyond. For example, in situ characterization of
physiological and genomic properties of animals could be achieved
through integration of sensors into these ultragentle actuators
(52, 53) and using deep neural networks to automate tasks such as
species identification (54). When combined with other existing
sampling mechanisms, such as deep sequencing on a small sample
from a single organism (55), in situ measurements and data collection
may enable biologists to better understand and conserve deep-sea
biodiversity.
MATERIALS AND METHODS
Fabrication of nanofiber-reinforced soft actuators
The objective of this study is to design and fabricate a gentle gripper
capable of nondestructive sampling of soft-bodied marine organisms.
To accomplish this goal, we developed a soft composite actuator
composed of a silicone rubber matrix and a flexible, yet tough,
nanofiber reinforcement layer. It is important to consider the
mechanical properties of silicone with respect to those of the target
organisms and in the context of the robot’s operational environment.
Although the elastic modulus of Dragon Skin 20 (measured as 0.37 MPa)
is an order of magnitude larger than that of jellyfish mesogleal tissue
(0.34 to 1.2 kPa) (19–22), the durometer of this silicone (Shore 20A)
provides durability and failure resistance under high applied
pressures (56).
Composite soft actuators were produced as per the approach
outlined in Fig. 2A. First, the lower portion of the actuator was
fabricated. Uncured silicone rubber (Dragon Skin 20, Smooth-On
Inc., Easton, PA) was poured into a custom-
designed 3D-printed mold, and the
assembly was degassed in a vacuum
chamber for 10 min. Next, a nanofiber
sheet was placed on the mold, enabling
the uncured silicone to permeate the
fabric. Nanofabric sheets [3 weight (wt)/
volume % nylon-6 (Nylon 6, Sigma-Aldrich,
St. Louis, MO)/3 wt/volume % poly-
urethane (McMaster Carr, Princeton, NJ)]
were manufactured using rotary jet
spinning, according to protocols discussed
in previous studies (35, 57, 58).
50 mm
For all tank and field studies, pristine
fiber sheets were embedded into soft
actuators at a fiber orientation angle of
0° (parallel to the longitudinal axis of the
actuator). The component was thermally
cured at 75°C for 15 min and removed
from the mold. Then, the upper portion
of the actuator (a thin membrane en-
closing the internal channel) was fabricated. A layer of uncured
silicone was generated using a film applicator, and the cured component
was placed onto this film. By cobonding the upper and lower parts
together, we formed the internal channel that was pressurized to
actuate each device.
Last, the components were thermally cured (75°C, 15 min), tubing
was inserted into the channel opening, and the area around the
tubing was sealed with epoxy (Sil-Poxy, Smooth-On Inc., Easton, PA).
For selected field tests, several drops of Smooth-On Silc Pig silicone
dye was added to the uncured Dragon Skin 20 during actuator
production; the purpose of this pigment was to improve the visual
contrast between the soft actuators and surrounding water. The
minute volume of dye added to the mixture did not affect thermal
curing of the silicone rubber.
Measuring actuator contact pressure
To gauge the contact pressure of our device, we measured the
blocked force exerted by the tip of each actuator (the part likely to
make contact with a target object) using a stationary 10-N load cell
(2530-10N, Instron, Norwood, MA) connected to a plate. Blocked
force represents the maximum force that can be generated by each
actuator. To minimize gravity effects, we mounted actuators vertically
with the distal tip pointing downward (fig. S2A). The distal end was
secured in a 3D-printed fixture, which was attached to the plate.
Each actuator was then pressurized hydraulically, and the blocked
force exerted by the actuator tip on the fixture was recorded using
Instron Bluehill 3 Testing Software (Instron 5544A, Norwood, MA).
Last, the contact pressure exerted by an actuator was calculated
using the area of the fixture and the measured force.
Laboratory evaluation of gripper performance
We evaluated the performance of our ultragentle robotic gripper
using a custom-built testing platform designed for repeated testing
of grasping operations underwater. The underwater environment
was a glass tank of about 60 cm wide by 180 cm long by 75 cm tall
filled with tap water. To position the gripper in 3D space, we mounted
a custom three-axis gantry above the tank, with an arm extending
down into the tank. The gripper was mounted to this arm and could
8 of 11
l
D
o
w
n
o
a
d
e
d
f
r
o
m
h
t
t
p
:
/
/
r
o
b
o
i
t
i
c
s
.
s
c
e
n
c
e
m
a
g
.
o
r
g
/
a
t
i
U
n
v
e
r
s
i
t
y
o
f
i
C
h
n
e
s
e
A
c
a
d
e
m
y
o
f
i
S
c
e
n
c
e
s
o
n
S
e
p
e
m
b
e
r
t
3
0
,
2
0
2
0
Sinatra et al., Sci. Robot. 4, eaax5425 (2019) 28 August 2019SCIENCE ROBOTICS | RESEARCH ARTICLE�











 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc