Medical Image Segmentation Using Deep Learning:
A Survey
Tao Lei, Senior Member, IEEE, Risheng Wang, Yong Wan, Xiaogang Du, Hongying Meng, Senior Member, IEEE
and Asoke K. Nandi, Fellow, IEEE
1
0
2
0
2
p
e
S
8
2
]
V
I
.
s
s
e
e
[
1
v
0
2
1
3
1
.
9
0
0
2
:
v
i
X
r
a
Abstract—Deep learning has been widely used for medical
image segmentation and a large number of papers has been
presented recording the success of deep learning in the field.
In this paper, we present a comprehensive thematic survey on
medical image segmentation using deep learning techniques. This
paper makes two original contributions. Firstly, compared to
traditional surveys that directly divide literatures of deep learning
on medical image segmentation into many groups and introduce
literatures in detail for each group, we classify currently popular
literatures according to a multi-level structure from coarse to fine.
Secondly, this paper focuses on supervised and weakly supervised
learning approaches, without including unsupervised approaches
since they have been introduced in many old surveys and they
are not popular currently. For supervised learning approaches,
we analyze literatures in three aspects: the selection of backbone
networks, the design of network blocks, and the improvement
of loss functions. For weakly supervised learning approaches, we
investigate literature according to data augmentation, transfer
learning, and interactive segmentation, separately. Compared
to existing surveys, this survey classifies the literatures very
differently from before and is more convenient for readers to
understand the relevant rationale and will guide them to think of
appropriate improvements in medical image segmentation based
on deep learning approaches.
Index Terms—medical
image segmentation, deep learning,
supervised learning, weakly supervised learning.
I. INTRODUCTION
M EDICAL image segmentation aims to make anatomical
or pathological structures changes in more clear in
images; it often plays a key role in computer aided diagnosis
and smart medicine due to the great improvement in diagnostic
efficiency and accuracy. Popular medical image segmentation
tasks include liver and liver-tumor segmentation [1, 2], brain
and brain-tumor segmentation [3, 4], optic disc segmentation
[5, 6], cell segmentation [7, 8], lung segmentation and pul-
monary nodules [9, 118], etc. With the development and pop-
ularization of medical imaging equipments, X-ray, Computed
Tomography (CT), Magnetic Resonance Imaging (MRI) and
ultrasound have become four important image assisted means
to help clinicians diagnose diseases, to evaluate prognopsis,
and to plan operations in medical institutions. In practical
applications, although these ways of imaging have advantages
as well as disadvantages,
they are useful for the medical
examination of different parts of human body.
To help clinicians make accurate diagnosis, it is necessary
to segment some crucial objects in medical images and extract
features from segmented areas. Early approaches to medical
image segmentation often depend on edge detection, template
matching techniques, statistical shape models, active contours,
and machine learning, etc. Zhao et al. [10] proposed a new
mathematical morphology edge detection algorithm for lung
CT images. Lalonde et al. [11] applied Hausdorff-based tem-
plate matching to disc inspection, and Chen et al. [12] also em-
ployed template matching to perform ventricular segmentation
in brain CT images. Tsai et al. [145] proposed a shape based
approach using horizontal sets for 2D segmentation of cardiac
MRI images and 3D segmentation of prostate MRI images.
Li et al. [146] used the activity profile model to segment
liver-tumors from abdominal CT images, while Li et al. [147]
proposed a framework for medical body data segmentation by
combining level sets and support vector machines (SVMs).
Held et al. [148] applied Markov random fields (MRF) to
brain MRI image segmentation. Although a large number of
approaches have been reported and they are successful in
certain circumstances, image segmentation is still one of the
most challenging topics in the field of computer vision due
to the difficulty of feature representation. In particular, it is
more difficult to extract discriminating features from medical
images than normal RGB images since the former often suffers
from problems of blur, noise, low contrast, etc. Due to the
rapid development of deep learning techniques [13], medical
image segmentation will no longer require hand-crafted feature
and convolutional neural networks (CNN) successfully achieve
hierarchical feature representation of images, and thus become
the hottest research topic in image processing and computer
vision. As CNNs used for feature learning are insensitive
to image noise, blur, contrast, etc., they provide excellent
segmentation results for medical images.
It is worth mentioning that there are currently two categories
of image segmentation tasks, semantic segmentation and in-
stance segmentation. Image semantic segmentation is a pixel-
level classification that assigns a corresponding category to
each pixel in an image. Compared to semantic segmentation,
the instance segmentation not only needs to achieve pixel-
level classification, but also needs to distinguish instances on
the basis of specific categories. In fact, there are few reports
on instance segmentation in medical image segmentation since
each organ or tissue is quite different. In this paper, we review
the advances of deep learning techniques on medical image
segmentation.
According to the number of labeled data, machine learning
is often categorized into supervised learning, weakly super-
vised learning, and unsupervised learning. The advantage of
supervised learning is that we can train models based on
carefully labeled data, but it is difficult to obtain a large
number of labeled data for medical images. On the contrary,
�
2
dom fields, k-means clustering, random forest, and reviewed
latest deep learning architectures such as the artificial neural
networks (ANNs), the convolutional neural networks (CNNs),
the recurrent neural networks (RNNs), etc. Tajbakhsh et al.
[19] reviewed solutions of medical image segmentation with
imperfect datasets, including two major dataset limitations:
scarce annotations and weak annotations. All these surveys
play an important role for the development of medical image
segmentation techniques. Hesamian et al. [119] reviewed on
three aspects of approaches (network structures),
training
techniques, and challenges. The network structures section
describes the main, popular network structures used for image
segmentation. The training techniques section discusses the J
Digit imaging technique used to train deep neural network
models. The challenges section describes the various chal-
lenges associated with medical image segmentation using deep
learning techniques. Meyer et al. [120] reviewed the advances
in the application or potential application of deep learning
to radiotherapy. Akkus et al. [149] provided an overview
of current deep learning-based segmentation approaches for
quantitative brain MRI images. Eelbode et al. [150] focus on
evaluating and summarizing the optimization methods used in
medical image segmentation tasks based primarily on Dice
scores or Jaccard indices.
Through studying the aforementioned surveys, researchers
can learn the latest techniques of medical image segmentation,
and then make more significant contributions for computer
aided diagnoses and smart healthcare. However, these sur-
veys suffer from two problems. One is that most of them
chronologically summarize the development of medical image
segmentation, and they thus ignore the technical branch of
deep learning for medical
image segmentation. The other
problem is that these surveys only introduce related tech-
nical development but not focus on the task characteristics
of medical image segmentation such as few-shot learning,
imbalance learning, etc., which limits the improvement of
medical image segmentation based on task-driven. To address
these two problems, we present a novel survey on medical
image segmentation using deep learning. In this work, we
make the following contributions:
1. We summarize the technical branch of deep learning for
medical image segmentation from coarse to fine as shown in
Fig. 1. The summation includes two aspects of supervised
learning and weakly supervised learning. The latest applica-
tions of neural architecture search (NAS), graph convolutional
networks (GCN), and multi-modality data fusion in medical
image analysis are also discussed. Compared to the previous
surveys, our survey follows conceptual developments and is
believed to be clearer.
2. On supervised learning approaches we analyze literature
from three aspects: the selection of backbone networks, the de-
sign of network blocks, and the improvement of loss functions.
This classification method can help subsequent researchers to
understand more deeply motivations and improvement strate-
gies of medical image segmentation networks. For weakly
supervised learning, we also review literatures from three
aspects for processing few-shot data or class imbalanced data:
data augmentation, transfer learning, and interactive segmen-
Fig. 1. An overview of deep learning methods on medical image segmentation
labeled data are not required for unsupervised learning, but the
difficulty of learning is increased. Weakly supervised learning
is between the supervised and unsupervised learning since it
only requires a small part of data labeled while most of data
are unlabeled.
Prior to the widespread application of deep learning, re-
searchers had presented many approaches based on model-
driven on medical image segmentation. Masood et al. [14]
made a comprehensive summary of many model-driven tech-
niques in medical image analysis, including image clustering,
region growing, and random forest. In [14], authors summa-
rized different segmentation approaches on medical images
according to different mathematical models. Recently, only a
few studies based on model-driven techniques were reported,
but more and more studies based on data-driven were reported
for medical image segmentation. In this paper, we mainly
focus on the evolution and development of deep learning
models on medical image segmentation.
In [15], Shen et al. presented a special review of the
application of deep learning in medical image analysis. This
review summarizes the progress of machine learning and
deep learning in medical
image registration, anatomy and
cell structure detection, tissue segmentation, computer-aided
disease diagnosis and prognopsis. Litjens et al. [16] reported
a survey of deep learning methods, the survey covers the
use of deep learning in image classification, object detection,
segmentation, registration and other tasks.
More recently, Taghanaki et al. [17] discussed the devel-
opment of semantic and medical image segmentation; they
categorized deep learning-based image segmentation solutions
into six groups, i.e., deep architectural, data synthesis-based,
loss function-based, sequenced models, weakly supervised,
and multi-task methods. To develop a more complete survey
on medical
image segmentation, Seo et al. [18] reviewed
classical machine learning algorithms such as Markov ran-
Supervised Learning Backbone Network Network BlockLoss FunctionSurveyWeakly Supervised LearningOthersData AugmentationTransfer LearningInteractive SegmentationNeural Architecture SearchGraph ConvolutionShape AttentiveMulti-modality Fusion�
3
end-to-end architectures, such as fully convolution network
(FCN) [20], U-Net [7], Deeplab [21], etc. In these structures,
an encoder is often used to extract image features while a
decoder is often used to restore extracted features to the
original image size and output the final segmentation results.
Although the end-to-end structure is pragmatic for medical
image segmentation, it reduces the interpretability of models.
The first high-impact encoder-decoder structure, the U-Net
proposed by Ronneberger et al. [7] has been widely used
for medical
image segmentation. Fig. 3 shows the U-Net
architecture.
U-Net: The U-Net solves problems of general CNN net-
works used for medical image segmentation, since it adopts
a perfect symmetric structure and skip connection. Different
from common image segmentation, medical images usually
contain noise and show blurred boundaries. Therefore, it is
very difficult to detect or recognize objects in medical images
only depending on image low-level features. Meanwhile, it is
also impossible to obtain accurate boundaries depending only
on image semantic features due to the lack of image detail
information. Whereas, the U-Net effectively fuses low-level
and high-level image features by combining low-resolution
and high-resolution feature maps through skip connections,
which is a perfect solution for medical image segmentation
tasks. Currently, the U-Net has become the benchmark for
most medical image segmentation tasks and has inspired a lot
of meaningful improvements.
tation. This orgnization is expected to be more conducive to
researchers in finding innovations for improving the accuracy
of medical image segmentation.
3. In addition to reviewing comprehensively the devel-
opment and application of deep learning in medical image
segmentation, we also collect the currently common public
medical
image segmentation datasets. Finally, we discuss
future research trends and directions in this field.
The rest of this paper is organized as follows. In Section
II, we review the development and evolution of supervised
learning applied to medical images, including the selection
of backbone network, the design of network blocks, and the
improvement of loss function. In Section III, we introduce
the application of unsupervised or weakly supervised methods
in the field of medical image segmentation and analyze the
commonly unsupervised or weakly supervised strategies for
processing few-shot data or class imbalanced data. In Section
IV, we briefly introduce some of the most advanced methods
of medical image segmentation, including NAS, application of
GCN, multi-modality data fusion, etc. In Section V, we collect
the currently available public medical
image segmentation
datasets, and summarize limitations of current deep learning
methods and future research directions.
II. SUPERVISED LEARNING
For medical image segmentation tasks, supervised learning
is the most popular method since these tasks usually require
high accuracy. In this section, we focus on the review of
improvements of neural network architectures. These improve-
ments mainly include network backbones, network blocks and
the design of loss functions. Fig. 2 shows an overview on
the improvement of network architectures based on supervised
learning.
Fig. 3. The U-Net architecture [7].
3D Net: In practice, as most of medical data such as CT and
MRI images exist in the form of 3D volume data, the use of
3D convolution kernels can better mine the high-dimensional
spatial correlation of data. Motivated by this idea, iek et al.
[22] extended U-Net architecture to the application of 3D
data, and proposed 3D U-Net that deals with 3D medical
data directly. Due to the limitation of computational resources,
the 3D U-Net only includes three down-sampling, which
cannot effectively extract deep-layer image features leading to
limited segmentation accuracy for medical images. In addition,
Milletari et al [23] proposed a similar architecture, V-Net, as
shown in Fig. 4. It is well known that residual connections
can avoid vanishing gradient and accelerate network conver-
gence, and it is thus easy to design deeper network structures
Fig. 2. An overview of network architectures based on supervised learning.
A. Backbone Networks
Image semantic segmentation aims to achieve pixel clas-
sification of an image. For this goal, researchers proposed
the encoder-decoder structure that is one of the most popular
Supervised Learning Backbone Network Network BlockLoss FunctionU-NetDepth SeparateDense ConnectionCascade 2D and 3DInceptionSkip ConnectionRecurrent Neural NetworkAttentionMultiscaleLocal Spatial AttentionChannel AttentionMixture AttentionNon-local AttentionPyramid PoolingAtrous Spacial Pyramid PoolingNon-Local and ASPPCross Entropy LossBoundary LossDice LossTversky LossExponential Logarithmic LossGeneralized Dice LossWeight Cross Entropy Loss3D NetConv 3×3,ReLUcopy and crop max pool 2×2up-conv 2×2Conv 1×1164641281282562565125121024102451251225625612812864642InputImagetileoutput segmentation map�
that can provide better feature representation. Compared to
3D U-Net, V-Net employs residual connections to design a
deeper network (4 down-samplings), and thus achieves higher
performance. Similarly, by applying residual connections to
3D networks, Yu et al. [24] presented Voxresnet, Lee et al.
[25] proposed 3DRUNet, and Xiao et al. [26] proposed Res-
UNet. However, these 3D Networks encounter same problems
of high computational cost and GPU memory usage due to a
very large number of parameters.
4
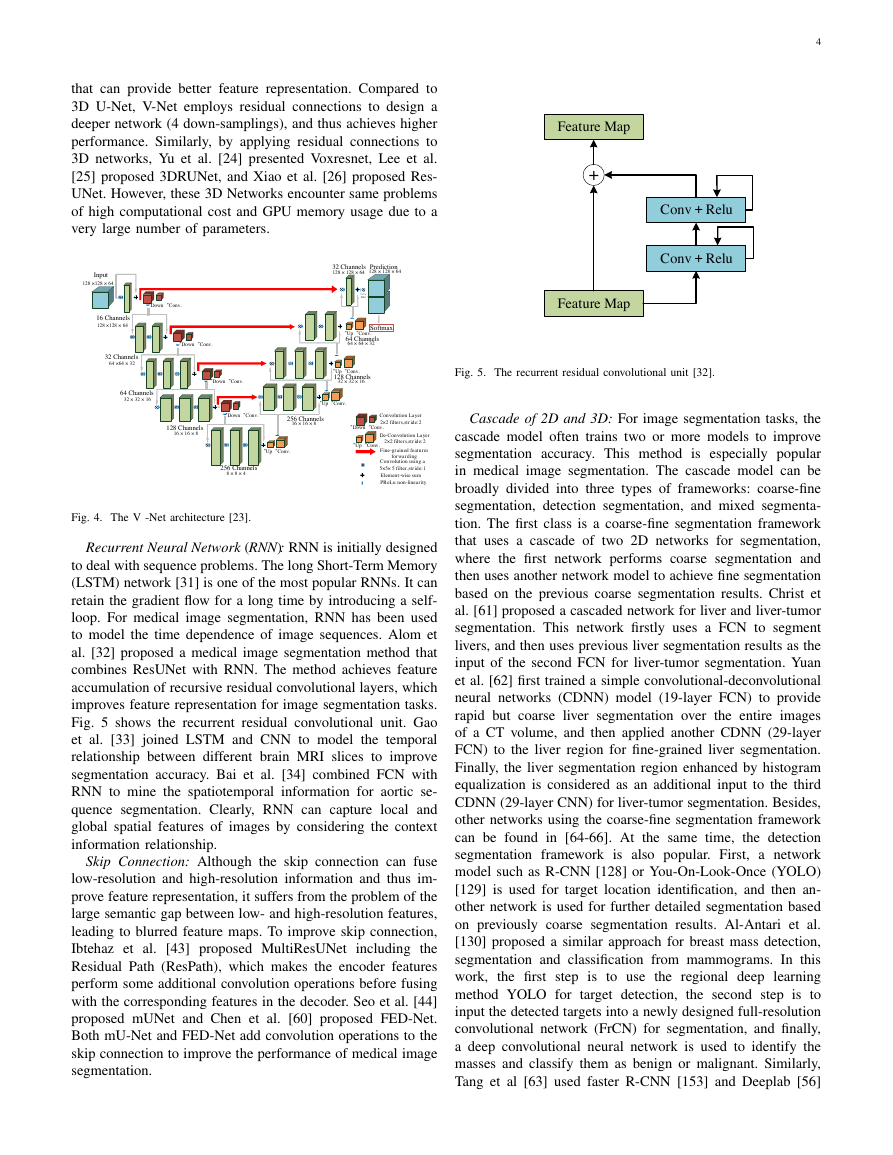
Fig. 5. The recurrent residual convolutional unit [32].
Cascade of 2D and 3D: For image segmentation tasks, the
cascade model often trains two or more models to improve
segmentation accuracy. This method is especially popular
in medical image segmentation. The cascade model can be
broadly divided into three types of frameworks: coarse-fine
segmentation, detection segmentation, and mixed segmenta-
tion. The first class is a coarse-fine segmentation framework
that uses a cascade of two 2D networks for segmentation,
where the first network performs coarse segmentation and
then uses another network model to achieve fine segmentation
based on the previous coarse segmentation results. Christ et
al. [61] proposed a cascaded network for liver and liver-tumor
segmentation. This network firstly uses a FCN to segment
livers, and then uses previous liver segmentation results as the
input of the second FCN for liver-tumor segmentation. Yuan
et al. [62] first trained a simple convolutional-deconvolutional
neural networks (CDNN) model (19-layer FCN) to provide
rapid but coarse liver segmentation over the entire images
of a CT volume, and then applied another CDNN (29-layer
FCN) to the liver region for fine-grained liver segmentation.
Finally, the liver segmentation region enhanced by histogram
equalization is considered as an additional input to the third
CDNN (29-layer CNN) for liver-tumor segmentation. Besides,
other networks using the coarse-fine segmentation framework
can be found in [64-66]. At the same time, the detection
segmentation framework is also popular. First, a network
model such as R-CNN [128] or You-On-Look-Once (YOLO)
[129] is used for target location identification, and then an-
other network is used for further detailed segmentation based
on previously coarse segmentation results. Al-Antari et al.
[130] proposed a similar approach for breast mass detection,
segmentation and classification from mammograms. In this
work,
the first step is to use the regional deep learning
method YOLO for target detection,
the second step is to
input the detected targets into a newly designed full-resolution
convolutional network (FrCN) for segmentation, and finally,
a deep convolutional neural network is used to identify the
masses and classify them as benign or malignant. Similarly,
Tang et al [63] used faster R-CNN [153] and Deeplab [56]
Fig. 4. The V -Net architecture [23].
Recurrent Neural Network (RNN): RNN is initially designed
to deal with sequence problems. The long Short-Term Memory
(LSTM) network [31] is one of the most popular RNNs. It can
retain the gradient flow for a long time by introducing a self-
loop. For medical image segmentation, RNN has been used
to model the time dependence of image sequences. Alom et
al. [32] proposed a medical image segmentation method that
combines ResUNet with RNN. The method achieves feature
accumulation of recursive residual convolutional layers, which
improves feature representation for image segmentation tasks.
Fig. 5 shows the recurrent residual convolutional unit. Gao
et al. [33] joined LSTM and CNN to model the temporal
relationship between different brain MRI slices to improve
segmentation accuracy. Bai et al. [34] combined FCN with
RNN to mine the spatiotemporal information for aortic se-
quence segmentation. Clearly, RNN can capture local and
global spatial features of images by considering the context
information relationship.
Skip Connection: Although the skip connection can fuse
low-resolution and high-resolution information and thus im-
prove feature representation, it suffers from the problem of the
large semantic gap between low- and high-resolution features,
leading to blurred feature maps. To improve skip connection,
Ibtehaz et al. [43] proposed MultiResUNet
including the
Residual Path (ResPath), which makes the encoder features
perform some additional convolution operations before fusing
with the corresponding features in the decoder. Seo et al. [44]
proposed mUNet and Chen et al. [60] proposed FED-Net.
Both mU-Net and FED-Net add convolution operations to the
skip connection to improve the performance of medical image
segmentation.
Input128 ×128 × 6416 Channels128 ×128 × 64“Down“Conv.“Down“Conv.“Down“Conv.“Down“Conv.32 Channels64 ×64 × 3264 Channels32 × 32 × 16128 Channels16 × 16 × 8256 Channels8 × 8 × 4“Up“Conv.“Up“Conv.“Up“Conv.“Up“Conv.256 Channels16 × 16 × 8128 Channels32 × 32 × 1664 Channels64 × 64 × 3232 Channels128 × 128 × 64Prediction128 × 128 × 641×1×filter“Down“Conv.“Up“Conv.Convolution Layer 2×2 filters,stride:2De-Convolution Layer 2×2 filters,stride:2Fine-grained features forwardingConvolution using a 5×5× 5 filter,stride:1Element-wise sumPReLu non-linearitySoftmaxFeature MapConv+ReluConv+Relu+Feature Map�
cascades for localization segmentation of the liver. In addition,
both Salehi et al [131] and Yan et al [132] proposed a kind
of cascade networks for whole-brain MRI and high-resolution
mammogram segmentation. This kind of cascade network can
effectively extract richer multi-scale context information by
using a posteriori probabilities generated by the first network
than normal cascade networks.
However, most of medical images are 3D volume data,
but a 2D convolutional neural network cannot learn temporal
information in the third dimension, and a 3D convolutional
neural network often requires high computation cost and
severes GPU memory consumption. Therefore some pseudo-
3D segmentation methods have been proposed. Oda et al [126]
proposed a three-plane method of cascading three networks
to segment the abdominal artery region effectively from the
medical CT volume. Vu et al. [127] applied the overlay of
adjacent slices as input to the central slice prediction, and
then fed the obtained 2D feature map into a standard 2D
network for model training. Although these pseudo-3D ap-
proaches can segment object from 3D volume data, they only
obtain limited accuracy improvement due to the utilization of
local temporal information. Compared to pseudo-3D networks,
hybrid cascading 2D and 3D networks are more popular.
Li et al. [67] proposed a hybrid densely connected U-Net
(H-DenseUNet) for liver and liver-tumor segmentation. This
method first employs a simple Resnet to obtain a rough liver
segmentation result, utilizing the 2D DenseUNet to extract
2D image features effectively, then uses the 3D DenseUNet
to extract 3D image features, and finally designs a hybrid
feature fusion layer to jointly optimize 2D and 3D features.
Although the H-DenseUNet reduces the complexity of models
compared to an entire 3D network, the model is complex and
it still suffers from a large number of parameters from 3D
convolutions. For the problem, Zhang et al. [37] proposed a
lightweight hybrid convolutional network (LW-HCN) with a
similar structure to the H-DenseUNet, but the former requires
fewer parameters and computational cost than the latter due
to the design of the depthwise and spatiotemporal separate
(DSTS) block and the use of 3D depth separable convolution.
Similarly, Dey et al. [68] also designed a cascade of 2D and
3D network for liver and liver-tumor segmentation.
Obviously, among the three types of cascade networks
mentioned above, the hybrid 2D and 3D cascade network
can effectively improve segmentation accuracy and reduce the
learning burdens.
In contrast to the above cascade networks, Valanarasu et
al. [133] proposed a complete cascade network namely KiU-
Net to perform brain dissection segmentation. The perfor-
mance of vanilla U-Net is greatly degraded when detecting
smaller anatomical structures with fuzzy noise boundaries.
To overcome this problem, authors designed a novel over-
complete architecture Ki-Net, in which the spatial size of the
intermediate layer is larger than that of the input data, and
this is achieved by using an up-sampling layer after each
conversion layer in the encoder. Thus the proposed Ki-Net
possesses stronger edge capture capability compared to U-Net
and finally it is cascaded with the vanilla U-Net to improve
the overall segmentation accuracy. Since the KiU-Net can
5
exploit both the low-level fine edges feature maps using Ki-
Net and the high-level shape feature maps using U-Net, it
not only improves segmentation accuracy but also achieves
fast convergence for small anatomical landmarks and blurred
noisy boundaries.
Others:A generating adversarial networks (GAN) [79] has
been widely used in many areas of computer vision. In its
infancy, the GAN was often used for data augmentation by
generating new samples, which would be reviewed in Section
III, but later researchers discovered that the idea of generative
confrontation could be used in almost any field, and was
therefore also used for image segmentation. Since medical
images usually show low contrast, blurred boundaries between
different tissues or between tissues and lesions, and sparse
medical image data with labels, U-Net-based segmentation
methods using pixel loss to learn local and global relation-
ships between pixels are not sufficient for medical
image
segmentation, and the use of generative adversarial networks
is becoming a popular idea for improving image segmentation.
Luc et al. [121] firstly applied the generative adversarial
network to image segmentation, where the generative network
is used for segmentation models and the adversarial network is
trained as a classifier. Singh et al. [122] proposed a conditional
generation adversarial network (cGAN) to segment breast
tumors within the target area (ROI) in mammograms. The
generative network learns to identify tumor regions and gen-
erates segmentation results, and the adversarial network learns
to distinguish between ground truth and segmentation results
from the generative network, thereby enforcing the generative
network to obtain labels as realistic as possible. The cGAN
works fine when the number of training samples is limited.
Conze et al. [123] utilized cascaded pretrained convolutional
encoder-decoders as generators of cGAN for abdominal multi-
organ segmentation, and considered the adversarial network as
a discriminator to enforces the model to create realistic organ
delineations.
In addition, the incorporation of the prior knowledge about
organ shape and position may be crucial for improving medical
image segmentation effect, where images are corrupted and
thus contain artefacts due to limitations of imaging techniques.
However, there are few works about how to incorporate prior
knowledge into CNN models. As one of the earliest studies
in this field, Oktay et al. [124] proposed a novel and general
method to combine a priori knowledge of shape and label
structure into the anatomically constrained neural networks
(ACNN) for medical image analysis tasks. In this way, the neu-
ral network training process can be constrained and guided to
make more anatomical and meaningful predictions, especially
in cases where input image data is not sufficiently informative
or consistent enough (e.g., missing object boundaries). Sim-
ilarly, Boutillon et al. [125] incorporated anatomical priors
into a conditional adversarial framework for scapula bone
segmentation, combining shape priors with conditional neural
networks to encourage models to follow global anatomical
properties in terms of shape and position information, and
to make segmentation results as accurate as possible. The
above study shows that improved models can provide higher
segmentation accuracy and they are more robust since priori
�
6
2) Inception: For CNNs, deep networks often give better
performances than shallow ones, but they encounter some new
problems such as vanishing gradient, the difficulty of network
convergence, the requirement of large memory usage, etc.
The inception structure overcomes these problems. It gives
better performance by merging convolution kernels in parallel
without increasing the depth of networks. This structure is able
to extract richer image features using multi-scale convolution
kernels, and to perform feature fusion to obtain better feature
representation. Inspired by GoogleNet [29-30], Gu et al.
[28] proposed CE-Net by introducing the inception structure
into medical image segmentation. The CE-Net adds atrous
convolution to each parallel structure to extract features on
a wide reception field, and adds 1 × 1 convolution of feature
maps, Fig. 8 shows the architecture of the inception. However,
the inception structure is complex leading to the difficulty of
model modification.
knowledge constraints are employed in the training process of
neural networks.
B. Network Function Block
1) Dense Connection: Dense connection is often used to
construct a kind of special convolution neural networks. For
dense connection networks, the input of each layer comes from
the output of all previous layers in the process of forward
transmission. Inspired by the dense connection, Guan et al.
[27] proposed an improved U-Net by replacing each sub-
block of U-Net with a form of dense connections as shown in
Fig. 6. Although the dense connection is helpful for obtaining
richer image features, it often reduces the robustness of feature
representation to a certain extent and increases the number of
parameters.
Zhou et al. [41] connected all U-Net layers (from one to
four) together as shown in Fig. 7. The advantage of this
structure is that it allows the network to learn automatically
importance of features at different layers. Besides, the skip
connection is redesigned so that features with different se-
mantic scales can be aggregated in the decoder, resulting in a
highly flexible feature fusion scheme. The disadvantage is that
the number of parameters is increased due to the employment
of dense connection. Therefore, a pruning method is integrated
into model optimization to reduce the number of parameters.
Meanwhile, the deep supervision [42] is also employed to
balance the decline of segmentation accuracy caused by the
pruning.
Fig. 6. Dense connection architecture [27].
Fig. 8. The inception architecture [28].
3) Depth Separability: To improve the generalization ca-
pability of network models and to reduce the requirement
of memory usage, many researchers focus on the study of
lightweight network models for complex medical 3D volume
data. By extending the depth separable convolution to the
design of 3D networks, Lei et al. [35] proposed a lightweight
V-Net (LV-Net) with fewer operations than V-Net for liver
segmentation. Generally, the depth separability decomposes
standard convolution into channel-wise convolution and point-
wise convolution [36]. The number of vanilla convolution
operation is usually DK × DK × M × N, where M is the
dimension of the input feature map, N is the dimension of
the output feature map, DK is the size of the convolution
kernel. However,
the number of the channel convolution
operation is DK × DK × 1 × M and the point convolution
is 1 × 1 × M × N. Compared to vanilla convolution,
the
computational cost of depthwise separable convolution is (1/N
+ 1/D2
K) times that of the vanilla convolution. Besides, Zhang
et al. [37] and Huang et al. [38] also proposed the application
of depthwise separable convolutions to the segmentation of
Fig. 7. The U-Net++ architecture [41].
1×1 conv+BN+Relu3×3 conv+BN+ReluConcatenation connectionX0,0X0,1X0,2X0,3X0,4X1,0X1,1X1,2X1,3X2,0X2,1X2,2X3,0X3,1X4,0Total LossDown-samplingUp-samplingSkip connectionXi,jConvolutionFeature MapFeature Map3×3 ConvRate = 1Channel = 5121×1 ConvRate = 1Channel = 5123×3 ConvRate = 3Channel = 5121×1 ConvRate = 1Channel = 5123×3 ConvRate = 3Channel = 5123×3 ConvRate = 1Channel = 5121×1 ConvRate = 1Channel = 5123×3 ConvRate = 5Channel = 5123×3 ConvRate = 3Channel = 5123×3 ConvRate = 1Channel = 512�
3D medical volume data. Other related works for lightweight
deep networks can be found in [39-40].
4) Attention Mechanism: For neural networks, an attention
block can selectively change input or assigns different weights
to input variables according to different importance. In recent
years, most of researches combining deep learning and visual
attention mechanism have focused on using masks to form
attention mechanisms. The principle of masks is to design
a new layer that can identify key features from an image,
through training and learning, and then let networks only focus
on interesting areas of images.
Local Spatial Attention: The spatial attention block aims
to calculate the feature importance of each pixel in space-
domain and extract the key information of an image. Jaderberg
et al. [45] early proposed a spatial transformer network (ST-
Net) for image classification by using spatial attention that
transforms the spatial information of an original image into
another space and retains the key information. Normal pooling
is equivalent to the information merge that easily causes the
loss of key information. For this problem, a block called spatial
transformer is designed to extract key information of images
by performing a spatial transformation. Inspired by this, Oktay
et al. [46] proposed attention U-Net. The improved U-Net
uses an attention block to change the output of the encoder
before fusing features from the encoder and the corresponding
decoder. The attention block outputs a gating signal to control
feature importance of pixels at different spatial positions. Fig.
9 shows the architecture. This block combines the Relu and
sigmoid functions via 1 × 1 convolution to generate a weight
map that is corrected by multiplying features from the encoder.
Fig. 9. The attention block in the attention U-Net [46].
Channel Attention: The channel attention block can achieve
feature recalibration, which utilizes learned global information
to emphasize selectively useful features and suppress useless
features. Hu et al. [47] proposed SE-Net that introduced the
channel attention to the field of image analysis and won
the ImageNet Challenge in 2017. This method implements
attention weighting on channels using three steps; Fig. 10
shows this architecture. The first is the squeezing operation,
the global average pooling is performed on input features to
obtain the 1 × 1 × Channel feature map. The second is the
excitation operation, where channel features are interacted to
reduce the number of channels, and then the reduced channel
features are reconstructed back to the number of channels.
Finally the sigmoid function is employed to generate a feature
weight map of [0, 1] that multiplies the scale back to the
original input feature. Chen et al. [60] proposed FED-Net that
uses the SE block to achieve the feature channel attention.
Mixture Attention: Spatial and channel attention mecha-
nisms are two popular strategies for improving feature rep-
7
Fig. 10. The channel attention in the SE-Net [47].
resentation. However, the spatial attention ignores the differ-
ence of different channel information and treats each channel
equally. On the contrary, the channel attention pools global
information directly while ignoring local information in each
channel, which is a relatively rough operation. Therefore, com-
bining advantages of two attention mechanisms, researchers
have designed many models based on a mixed domain atten-
tion block. Kaul et al. [48] proposed the focusNet using a
mixture of spatial attention and channel attention for medical
image segmentation, where the SE-Block is used for channel
attention and a branch of spatial attention is designed. Besides,
other related works can be found in [39-40].
To improve the feature discriminant representation of net-
works, Wang et al. [53] embedded an attention block inside
the central bottleneck between the contraction path and the
expansion path of the U-Net, and proposed the ScleraSegNet.
Furthermore, they compared the performance of channel at-
tention, spatial attention, and different combinations of two
attentions for medical image segmentations. They concluded
that the channel-centric attention was the most effective in
improving image segmentation performance. Based on this
conclusion, they finally won the championship of the sclera
segmentation benchmarking competition (SSBC2019).
Although those attention mechanisms mentioned above im-
prove the final segmentation performance, they only perform
an operation of local convolution. The operation focuses on the
area of neighboring convolution kernels but misses the global
information. In addition, the operation of down-sampling leads
to the loss of spatial information, which is especially unfavor-
able for biomedical image segmentation. A basic solution is to
extract long-distance information by stacking multiple layers,
but this is low efficiency due to a large number of parameters
and high computational cost. In the decoder, the up-sampling,
the deconvolution, and the interpolation are also performd in
the way of local convolution.
Non-local Attention: Recently, Wang et al. [54] proposed a
Non-local U-Net to overcome the drawback of local convo-
lution for medical image segmentation. The Non-local U-Net
employs the self-attention mechanism and the global aggrega-
tion block to extract full image information during the parts
of both up-sampling and down-sampling, which can improve
the final segmentation accuracy. Fig. 11 shows the global
aggregation block. The Non-local block is a general-purpose
block that can be easily embedded in different convolutional
neural networks to improve their performance.
It can be seen that the attention mechanism is effective
for improving image segmentation accuracy. In fact, spatial
attention looks for interesting target regions while channel
1×1 Conv1×1 Conv+Relu1×1 ConvSigmoidResampler×HWCHWC1×1×C1×1×CF(gp)F(ex)F(scale)�
attention looks for interesting features. The mixed attention
mechanism can take advantages of both spaces and channles.
However, compared with the non-local attention, the conven-
tional attention mechanism lacks the ability of exploiting the
associations between different targets and features, so CNNs
based on non-local attention usually exhibit better performance
than normal CNNs for image segmentation tasks.
5) Multi-scale Information Fusion: One of the challenges
in medical image segmentation is a large range of scales
among objects. For example, a tumor in the middle or late
stage could be much larger than that in the early stage. The
size of perceptive field roughly determines how much context
information we can use. The general convolution or pooling
only employs a single kernel, for instance, a 3 × 3 kernel for
convolution and a 2 × 2 kernel for pooling.
Pyramid Pooling: The parallel operation of multi-scale pool-
ing can effectively improve context information of networks,
and thus extract richer semantic information. He et al. [55]
first proposed spatial pyramid pooling (SPP) to achieve multi-
scale feature extraction. The SPP divides an image from the
fine space to the coarse space, then gathers local features and
extracts multi-scale features. Inspired by the SPP, a multi-scale
information extraction block is designed and named residual
multi-kernel pooling (RMP) [28] that uses four pooling kernels
with different sizes to encode global context
information.
However, the up-sampling operation in RMP cannot restore the
loss of detail information due to pooling that usually enlarges
the receptive field but reduces the image resolution.
Atrous Spatial Pyramid Pooling: In order to reduce the
loss of detail information caused by pooling operation, re-
searchers proposed atrous convolution instead of the polling
operation. Compared with the vanilla convolution, the atrous
convolution can effectively enlarge the receptive field without
increasing the number of parameters. Combining advantages
of the atrous convolution and the SPP block, Chen et al. [56]
proposed the atrous spatial pyramid pooling module (ASPP) to
improve image segmentation results. The ASPP shows strong
recognition capability on same objects with different scales.
Similarly, Lopez et al. [57] applied the superposition of multi-
scale atrous convolutions to brain tumor segmentation, which
achieves a clear accuracy improvement.
However, the ASPP suffers from two serious problems for
image segmentation. The first problem is the loss of local
information as shown in Fig. 12, where we assume that the
convolutional kernel is 3×3 and the dilation rate is 2 for three
iterations. The second problem is that the information could
be irrelevant across large distances. How to simultaneously
handle the relationship between objects with different scales is
important for designing a fine atrous convolutional network. In
response to the above problems, Wang et al. [58] designed an
hybrid expansion convolution (HDC) networks. This structure
uses a sawtooth wave-like heuristic to allocate the dilation rate,
so that information from a wider pixel range can be accessed
and thus the gridding effect is suppressed. In [58], authors gave
several atrous convolution sequences using variable dilation
rate, e.g., [1,2,3], [3,4,5], [1,2,5], [5,9,17], and [1,2,5,9].
Non-local and ASPP: The atrous convolution can effi-
ciently enlarge the receptive field to collect richer semantic
8
it
information, but it causes the loss of detail information due
to the gridding effect. Therefore,
is necessary to add
constraints or establish pixel associations for improving the
atrous convolution performance. Recently, Yang et al. [59]
proposed a combination block of ASPP and Non-local for the
segmentation of human body parts, as shown in Fig. 13. ASPP
uses multiple parallel atrous convolutions with different scales
to capture richer information, and the Non-local operation
captures a wide range of dependencies. This combination
possesses advantages of both ASPP and Non-local, and it has
a good application prospect for medical image segmentation.
C. Loss Function
In addition to the design of both network backbone and
the function block, the selection of loss functions is also an
important factor for the improvement of network performance.
1) Cross Entropy Loss: For image segmentation tasks, the
cross entropy is one of the most popular loss functions. The
function compares pixel-wisely the predicted category vector
with the real segmentation result vector. For the case of binary
segmentation, let P (Y = 1) = p and P (Y = 0) = 1 − p,
then the prediction is given by the sigmoid function, where
P ( ˆY = 1) = 1/(1 + e−x) = ˆp and P ( ˆY = 0) = 1 − 1/(1 +
e−x) = 1 − ˆp, x is the output of neural networks. The cross
entropy loss is defined as
CE(p, ˆp) = −(plog(ˆp) + (1 − p)log(1 − ˆp)).
(1)
2) Weighted Cross Entropy Loss: The cross entropy loss
deals with each pixel of images equally, and thus outputs an
average value, which ignores the class imbalance and leads
to a problem that
the loss function depends on the class
including the maximal number of pixels. Therefore, the cross
entropy loss often shows low performance for small target
segmentation.
To address the problem of class imbalance, Long et al. [20]
proposed weighted cross entropy loss (WCE) to counteract
the class imbalance. For the case of binary segmentation, the
weighted cross entropy loss is defined as
W CE(p, ˆp) = −(βplog(ˆp) + (1 − p)log(1 − ˆp)),
(2)
where β is used to tune the proportion of positive and negative
samples, and it is an empirical value. If β > 1, the number of
false negatives will be decreased; on the contrary, the number
of false positives will be decreased when β < 1. In fact, the
cross entropy is a special case of the weighted cross entropy
when β = 1. To adjust the weight of positive and negative
samples simultaneously, we can use the balanced cross entropy
(BCE) loss function that is defined as
BCE(p, ˆp) = −(βplog(ˆp) + (1 − β)(1 − p)log(1 − ˆp)).
(3)
In [7], Ronneberger et al. proposed U-Net in which the
cross entropy loss function is improved by adding a distance
function. The improved loss function is able to improve the
learning capability of models for inter-class distance. The
distance function is defined as
D(x) = ω0e
−(d1(x)+d2(x)2
2σ2
,
(4)
�















 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc