Journal of Power Sources 155 (2006) 401–414
Review
Safety mechanisms in lithium-ion batteries
P.G. Balakrishnan, R. Ramesh, T. Prem Kumar
∗
Electrochemical Power Systems Division, Central Electrochemical Research Institute, Karaikudi 630006, Tamil Nadu, India
Received 18 October 2005; received in revised form 1 December 2005; accepted 2 December 2005
Available online 28 February 2006
Abstract
With increasing use of lithium-ion power packs, reports of occasional incidents of severely debilitating and sometimes fatal tragedies appear in
the news. This review analyzes possible scenarios that trigger such hazards before proceeding to discuss safety mechanisms such as pressure release
valves, one-shot fuses, reversible and irreversible positive temperature coefficient elements, shutdown separators, chemical shuttles, non-flammable
electrolytes and coatings.
© 2006 Published by Elsevier B.V.
Keywords: Lithium-ion batteries; Safety; Battery hazard; Non-flammable electrolytes; Thermal runaway
Contents
4.
5.
6.
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1.
2.
Lithium-ion battery hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3. Conventional safety devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Safety vents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3.1.
3.2.
Thermal fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3.3. Other circuit breakers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Self-resetting devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4.1. Ceramic PTC materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4.2. Conductive-polymer PTC devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Shutdown separators. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Electrolytes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.1. Non-flammable electrolytes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.2. Redox shuttles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.2.1. Halide shuttles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.2.2. Metallocene shuttles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.2.3. Aromatic redox shuttles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Shutdown additives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Ionic liquids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Electrolyte salts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
LiPF3(C2F5)3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.5.1.
LiN(SO2CF3)2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.5.2.
6.5.3.
LiBC4O8 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7. Active materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7.1. Carbon anode. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7.2.
LiCoO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.3.
6.4.
6.5.
402
402
403
403
404
404
404
405
405
406
406
407
407
407
408
408
408
408
409
409
409
409
409
410
410
∗
Corresponding author. Tel.: +91 4565 227888/227550–9; fax: +91 4565 227779.
E-mail addresses: premlibatt@yahoo.com, prem@cecri.res.in (T. Prem Kumar).
0378-7753/$ – see front matter © 2006 Published by Elsevier B.V.
doi:10.1016/j.jpowsour.2005.12.002
�
402
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
7.3.
7.4.
7.5.
LiNiO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
LiMn2O4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
LiFePO4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
8. Coatings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
9. Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
410
410
411
411
411
411
1. Introduction
Perhaps the word lithium itself has questions of safety tagged
to it. In fact, safety is a recurring theme even with lithium-ion
cells where metallic lithium is replaced with lithium-insertion
active materials. Ridden with a poor understanding of the
fledgling lithium-ion battery technologies, what manufactur-
ers and consumers fear are accidents during use or inadvertent
abuse. For example, in an incident that occurred at Apple in
1995, lithium-ion batteries got overcharged during an in-house
testing of a newly manufactured PowerBook 5300 portable com-
puter [1]. Apple then removed all lithium-ion power packs from
their product lines [2]. Hereabouts, Ericsson announced that
its mobile phones and other portable electronic applications
would wean away from lithium-ion batteries [3]. In fact, several
other OEM manufacturers have also been proactive in recalling
their products. In 2000, in cooperation with the U.S. Consumer
Product Safety Commission, Dell voluntarily recalled 27,000
lithium-ion batteries, manufactured by Sanyo Electric Co. Ltd.,
and sold in notebook computers. Compaq also recalled 55,000
notebook lithium-ion batteries manufactured by Sony Corpora-
tion because of a defect in the circuit board that controls the
recharge and discharge processes. One of the recent lithium-ion
battery recalls with the USPSC was in 2002 when, upon receiv-
ing five reports of batteries overheating (in three of the instances
they caught fire), EV Global Motors Company announced the
recall of 2000 batteries in their electric bicycles.
Withdrawal of products, loss of market and even a ban on
lithium-ion batteries were part of a backlash prompted by these
incidents. Thus arose the need for safety in commercial lithium-
ion battery applications. Today, lithium-ion batteries are the
state-of-the-art power sources for a variety of portable electronic
devices. They combine high energy density and excellent cycle
life, and have no memory effect. That no lithium battery-related
accident has been reported in the recent past is testimony to
improved safety characteristics of present-day lithium battery
products. The excellent safety record has been brought about
by regulations governing the safety of the cells [4]. Continual
improvements in safety are being made especially with large
battery packs as for electric traction and load leveling [5]. The
gravity of the situation becomes evident considering the market
share for lithium batteries. Of the US$ 37 billion battery market
in 2000, about US$ 2.9 billion was shared by lithium batteries,
the share for primary and secondary lithium batteries being US$
1.1 and 1.8 billion, respectively [6]. According to a prediction
rechargeable lithium battery market should grow to more than
US$ 2.1 billion by 2009 [7].
Lithium-ion batteries combine highly energetic materials in
contact with a flammable electrolyte based on organic solvents.
They can suffer premature failure if subjected to conditions for
which they are never designed. Any abuse, including dispos-
ing in fire, overcharging, external short circuiting or crushing,
can trigger spontaneous heat-evolving reactions, which can lead
to fire and explosion. Lithium-ion batteries must pass a num-
ber of safety tests before they can be certified for use by a
consumer. The tests include electrical tests such as external
short circuit, mechanical tests such as nail penetration, crush-
ing, dropping to the ground, and environmental tests such as
heating in a microwave oven, throwing into a hot liquid, and
leak tests in a vacuum. Several techniques have been devised to
improve safety. They include use of safety vents, positive tem-
perature coefficient (PTC) elements, shutdown separators, more
oxidation-tolerant or less flammable electrolyte constituents and
redox shuttle mechanisms. In this paper we review safety mech-
anisms adopted in commercial lithium-ion batteries.
2. Lithium-ion battery hazards
Apart from the fact that lithium batteries have highly oxidiz-
ing and reducing materials, their safety is compounded by the
fact that the design of these non-aqueous cells has an inherent
drawback of poor heat dissipation. Compared to lithium metal-
anode batteries, lithium-ion cells are considered to be safer.
The redox potentials of metallic lithium and lithiated carbons
(LixC6), for example, are similar. The reactive surface area of
the carbonaceous anode with a typical particle size of about
10 m is large. Although the specific surface area of the lithi-
ated carbon electrode has been demonstrated to increase by a
factor of five upon cycling [8], the reactivity of anode is kineti-
cally limited by the slow transport of lithium from the galleries to
the surface of the graphitic electrode [9–11]. Another important
factor that contributes to enhanced safety of lithium-ion batteries
vis-`a-vis lithium metal anode batteries is the much higher melt-
ing point of LixC6 as compared to that of lithium metal. The
low melting point of lithium (180
C) poses an additional risk of
fire hazard from molten lithium generated by inadvertent over-
heating. However, exothermic reactions between LixC6 and the
electrolyte can be triggered by the application of heat [12,13].
The potential ranges experienced in common 4-V lithium-
ion cells are beyond the thermodynamic stability windows of
the electrolytes. Electrolytes, therefore, decompose upon con-
tact with the charged active materials, both anodes [14–19] and
cathodes [20–24]. The interface between the cathode and the
electrolyte is further complicated by partial dissolution of the
positive active materials [25–27]. This is particularly a problem
at the end of charging and at elevated temperatures, conditions
under which electrolyte oxidation can proceed at accelerated
rates [28–34].
◦
�
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
403
◦
The temperature of a cell is determined by the heat balance
between the amount of heat generated and that dissipated by the
cell. When a cell gets heated above a certain temperature (usu-
ally above 130–150
C), exothermic chemical reactions between
the electrodes and electrolyte set in, raising its internal temper-
ature. If the cell can dissipate this heat, its temperature will
not rise abnormally. However, if the heat generated is more than
what can be dissipated, the exothermic processes would proceed
under adiabatic-like conditions and the cell’s temperature will
increase rapidly. The rise in temperature will further accelerate
the chemical reactions, rather than the desired galvanic reactions,
causing even more heat to be produced, eventually resulting in
thermal runaway [9,35,36], whose onset temperature determines
the safety limit of the device. Any pressure generated in these
processes can cause mechanical failures within cells, triggering
short circuits, premature death of the cell by irreversible inter-
ruptions in the current path, distortion, swelling and rupture of
cell casing.
It is clear that the thermal stability of batteries depends on its
ability to dissipate the heat. The ability of an object to absorb
heat is defined by its thermal capacity. Obviously, for a given
amount of heat, bigger and heavier objects would suffer less
temperature rise than would a similar object that is smaller and
lighter. Thus, for lithium-ion batteries, which are designed for
applications where size and weight are a premium, a decrease in
the thermal capacity is an unavoidable penalty. Thus, heat dissi-
pation in lithium-ion batteries turns out to be a major engineering
challenge, especially for those designed for high power appli-
cations. Designs for effective heat dissipation must be adopted
both at the cell and battery pack levels. Heat dissipation can
occur by convection and radiation at the surface of the cell. Heat
dissipation by convection depends, among other things, on the
external surface area and geometry of the cell. However, heat
dissipated by radiation depends on the nature of the surface
of the cell and makes up nearly 50% of the dissipation [37].
Radiation dissipation can be improved by use of cell cases that
have high thermal conductivity and labels that have high emis-
sivity. Thermal performance is rarely a cause for cell failure
in low-power cells that have simple designs. However, thermal
design of high-power cells is not that simple. Poor designs can
result in localized hotspots within the cell, which can lead to cell
failure.
Possible exothermic reactions that trigger thermal runaway
include [36,38]: (i) thermal decomposition of the electrolyte;
(ii) reduction of the electrolyte by the anode; (iii) oxidation of
the electrolyte by the cathode; (iv) thermal decomposition of the
anode and cathode; and (v) melting of the separator and the con-
sequent internal short. Moreover, high-voltage metal cathodes
are known to release oxygen at elevated temperatures [39,40].
Thermal runaway is often caused under abuse conditions, which
can be thermal (overheating), electrical (overcharge, high pulse
power) or mechanical (crushing, internal or external short cir-
cuit) [36,41].
It must be noted that the release of materials from batteries can
be benign, mild or violent. Battery hazards are classified accord-
ing to the damage they cause [35]. Physical hazards involve a
simple rupture of battery case; chemical hazards result from
leakage or venting of corrosive or toxic materials in the bat-
tery; both chemical and physical hazards can cause equipment
damage due to breakage or corrosion of electrical/electronic
components; environmental hazards arise from the reactive and
flammable nature of lithium and/or leakage of toxic materials
from batteries that are improperly disposed.
An area that has often been overlooked is the possible embrit-
tlement of container metal with lithium (similar to hydrogen
embrittlement). This can happen if the metal in question is
capable of alloying with lithium. In such a case, a spontaneous
transfer of lithium to the alloying metal casing can occur [42].
This can lead to a structural destruction of the container mate-
rial, resulting in leakage paths. Lithium embrittlement at highly
stressed regions of battery containers can accelerate crack prop-
agation. Although lithium battery leakages have been observed,
no conclusive evidence is available to merit extensive research
in this direction.
3. Conventional safety devices
A predominant mechanism by which lithium batteries are
rendered safe involves limiting the current passing through them.
Current-limiting devices such as positive thermal coefficient
devices are designed to respond to high temperatures. Several
factors play a role in the operation of these devices: the ambient
temperature, thermal insulating properties of the container, heat
generated in the equipment, cumulative heat in the battery pack,
and rate and duration of discharge. Thus, it becomes necessary to
consult the manufacturer or conduct tests in order to determine
the suitability of a battery pack for a particular application.
Apart from preventing flow of excessive currents that can
potentially damage cells, current-limiting protection devices
must withstand continuous flow of the load’s design current
and tolerate normal surges and transients. Furthermore, safety
devices must also fit into very small spaces and must be rela-
tively cheap. For acceptance in commerce, the current-limiting
device must be fail-proof, which also means that it should not
be prone to false tripping, factors that can decide customer dis-
satisfaction. It must be pointed out that batteries regulated with
external electronic devices such as PTC elements and integrated
circuits would not only have higher manufacturing costs but also
lower energy density.
3.1. Safety vents
Conventional safety mechanisms include such devices as
vents and current-limiting devices like fuses and circuit break-
ers. Safety vents open in response to a sudden increase in cell
pressure, allowing gases to escape. If the pressure inside a cell
builds up, a plastic laminate membrane is punctured by a spike
incorporated in the vent in the cell top. A safe release of internal
pressure precludes dangerous rupture of the cell casing. Safety
vents can be designed to operate at pre-set internal cell tempera-
tures. Today, vents are a back-up safety device. During instances
of electrical abuse, other devices such as a positive temperature
coefficient device (described below) override the vent. If bat-
teries are subjected to severe mechanical abuse conditions, the
�
404
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
safety vent provides a means of releasing internal pressure and
prevents the cell from reaching excessively high temperatures.
Kato et al. [43] developed a safety mechanical link by which
a concave aluminum disk welded to the cathode would break
the circuit upon release of gas. In this design, lithium carbonate
deliberately added to the LiCoO2 cathode mix would decompose
to yield CO2 when the cell is overcharged to greater than 4.8 V.
The built-up pressure would push the aluminum disk, discon-
necting the cathode lead from the circuit. This simple mechanism
prevents the cell from the thermal runaway caused by an exces-
sive overcharge. Choi et al. [44] have shown that in addition to
providing safety, the added lithium carbonate can suppress the
initial irreversibility of the carbon anode.
Since the safety vent opens up the cell, spewing out a signif-
icant quantity of volatile organics, it is used as a back-up safety
device. In fact, other safety devices such as PTC elements over-
ride the safety vent during abuse. Under severe mechanical and
electrical abuse conditions, the vent provides a safe means of
releasing internal pressure before the cell reaches excessively
high temperatures.
3.2. Thermal fuses
The oldest and most common current limiter is the one-shot
fuse, which is a wire of a fusible alloy with resistance and ther-
mal characteristics that allow it to melt when a pre-set current
flows through it. Some fuses require several seconds to trip, but
they are inherently fast-acting. The advantages of the fuse as a
safety device lie in its simple construction, low cost and avail-
ability in a wide range of currents and voltages ranges. Fuses act
by destroying themselves, thereby positively and permanently
opening the circuits they protect. Thus, they must be replaced
once blown, which is another advantage (as it draws the atten-
tion of the user to take action for resuming service) although
the mechanical action involves labor. However, fuses can pre-
maturely blow under other conditions such as pulse discharges
(or repeated pulse discharges that can degrade the alloy), which
are normal operational modes of batteries. Moreover, there is the
possibility of inadvertent replacement with fuses with higher or
lower current ratings, which can result in improper use of equip-
ment. Fuses are wired in series with the cell stack and will open
when a pre-set cell temperature is reached. Thermal fuses are
employed as protection against thermal runaway and are usually
set to open at 30–50
C above the maximum operating temper-
ature of the battery. Fuses are cheap and are ideal for low-cost,
throwaway products with limited warranties.
◦
3.3. Other circuit breakers
Other circuit breakers such as magnetic switches, bimetallic
thermostats and electronic protection circuit modules can be
used to protect power packs and to monitor their temperature.
They must also tolerate continuous design current as that of
the load as well as occasional current surges, without tripping.
However, their size and cost often rule out the application of the
first two in many onboard circuits, especially where space is at
a premium.
Thermistors sense the internal temperature of the battery,
and provide information to an external control through a cali-
brated resistance. Thermistor controls may be located in a battery
charger. The thermistor is attractive as the control can be set to
meet specific conditions of charge and to regulate input current
to the battery. This device can also be used to control the bat-
tery through �T/�t control, where T and t are the temperature
and time, respectively. PTC thermistors have a positive temper-
ature coefficient, as will be described below. Similarly, thermis-
tors whose resistances decrease with increasing temperature are
called negative temperature coefficient (NTC) thermistors. Both
are used for monitoring and protection of control circuits.
The thermostat or temperature cut-off (TCO) devices oper-
ate at a fixed temperature, and can be used to terminate charge
(or discharge) when a pre-set internal battery temperature is
reached. TCOs are usually resettable. They are connected in
series with the cell stack.
Electronic safety circuits, commonly referred to as protection
circuit module (PCM), are usually attached to battery packs as
separate modules. In the event of a wrongful condition, such
as short circuit, the PCM opens the battery circuit and prevents
damage to the pack. Some groups believe that the cell chemistry
in lithium-ion cells can be modified and safety levels raised,
rendering PCMs redundant [45].
Unlike aqueous electrolyte cells, which have an inher-
ent balance-adjusting mechanism such as gas recombination,
lithium-ion cells require an external overcharge/overdischarge
protection system, particularly those for use in specialized appli-
cations as in electric traction and spacecraft. This can be pro-
vided through an electronic control circuit. However, the cost
component of the circuits is kept small as compared to the
cost of the batteries themselves. The basic circuitry consists
of a bypass circuit controlled by a microchip based on MOS-
FET. The bypass circuit gets activated when a cell in a pack
reaches a given state-of-charge/discharge earlier than other cells.
Thus, the charge/discharge process is terminated until balance is
regained. Open-circuit voltage of lithium-ion cells can be used
as indicators of their state-of-charge, electronic controllers can
be designed to sense voltages and, thereby, switch on or off
the charging/discharging circuit. This ensures charge balance
among cells in a pack and damage by overcharge/overdischarge
of individual cells. In specialized applications, battery packs
come with protection circuits that monitor cell temperature and
activate cooling gadgets such as fans.
4. Self-resetting devices
Factors such as inconvenience of replacement and prema-
ture failure of fuses (which call for time-consuming technical
services), unsuitability of integrating devices such as mag-
netic/thermal switches onboard, size restrictions and cost led
to a search for a self-resetting, fuse-like device. Thus, emerged
safety devices called positive temperature coefficient devices
based on materials whose resistance increases dramatically with
a rise in temperature. For example, if a large current flows across
the PTC element, as during external short circuiting, its temper-
ature rises up abruptly up due to Joule heat evolution within the
�
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
405
PTC element. A concomitant and abnormally high resistance of
the PTC element prevents current flow. Thus, upon activation,
the resistance of the PTC element shoots up, leading to a precip-
itous fall in the current, which limits heat generation in the cell.
Once the cause for alarm is removed, the cell and PTC element
cool and the resistance of the latter drops, allowing resumption
of charge/discharge. PTC elements are generally installed inside
cells. The temperature above which the resistance of the PTC
element jumps to an infinite value is called the “trip tempera-
ture,” whose value is generally set at about 100
C.
◦
Although the primary purpose of PTC devices is to protect
batteries against external short circuits, they also provide pro-
tection under certain other electrical abuse conditions. This is
accomplished by limiting current flow when the cell temperature
reaches the designed activating temperature of the PTC device.
For extended equipment life, the PTC must work reversibly.
Although PTC devices can operate in this way several times, it
will not reset indefinitely. Fortunately, when they cease to reset,
they remain in their high-resistance condition, rendering the cell
unusable. PTC devices usually come as surface-mountable units
and are compatible with pick-and-place equipment. Thus, they
carry little assembly-costs. But because they are costlier than
fuses, they become economically attractive only when used in
equipment that are costly or demand long-term warranties.
4.1. Ceramic PTC materials
Ceramic materials with fuse-like action were the materials
of choice for early PTC elements. Ceramic PTC devices can
operate under high voltages and can return to their normal resis-
tance mode with great accuracy. Thus, they are attractive for
application in several high-voltage circuits although their rela-
tively large sizes preclude their use in miniature high-component
density gadgets. It must be noted that their applicability in low-
voltage circuits is undermined by their high inherent resistance,
the high voltage drop across which can cause problems with
the operation of the gadget. Another intrinsic disadvantage with
ceramic PTC materials is their high thermal mass, which ren-
ders their reaction time to moderate over-currents longer than
those of the components in the gadget. The sluggish response
can damage costly equipment.
4.2. Conductive-polymer PTC devices
◦
Conductive-polymer PTC devices are non-linear PTC ther-
mistors based on a composite of polymers and conductive par-
ticles. It is known that above their glass transition temperatures
(Tg) polymers transform into an amorphous state and return to
their crystalline state upon cooling to temperatures below their
Tg. At normal operating temperatures, the conductive particles
embedded in a crystalline polymer matrix provide a low resis-
tance path for current flow. At elevated temperatures (typically
∼125
C), the polymer’s structure changes to an amorphous
state. The accompanying expansion of the matrix breaks the
conductive pathway between the embedded particles, rapidly
increasing the device’s resistance by several orders (Fig. 1). This
reduces the current to a relatively low and safe level. An advan-
tage of PTC devices is that this trickle current maintains the
internal temperature of the cell high, prevents the conductive
chains from returning to their original state. In other words,
the trickle current “latches” the PTC device in its tripped state.
Upon opening the circuit the device cools, allowing the polymer
matrix to return to its normal state and returns the resistance of
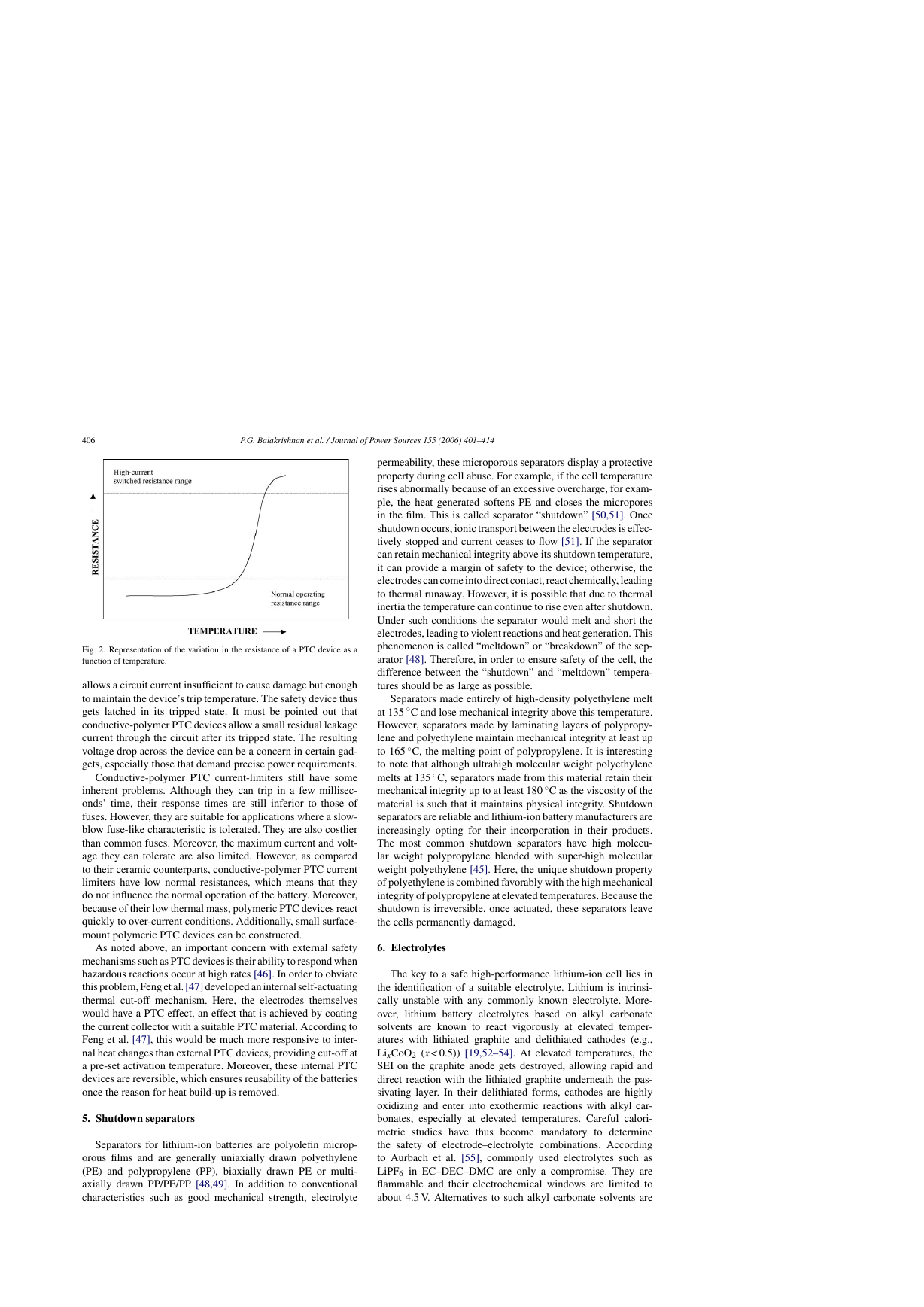
the device to its normal low value. Fig. 2 shows the variation of
the resistance of a PTC device as a function of temperature.
Conductive-polymer PTC devices are made from a blend
of plastics and conductive materials. The temperature of the
conducting-polymer PTC device is determined by the ambient
temperature and heat generated by internal I2R losses. Under
normal operating conditions, the I2R losses are too low to gen-
erate enough heat to transform the polymer into its amorphous
state. However, under abuse conditions when large currents flow
through the device, the I2R losses become sufficiently high,
increasing the temperature and hence the resistance of the PTC
element. The reduction in the current in turn reduces the I2R
losses. Upon regaining thermal equilibrium, the PTC device
Fig. 1. Principle of a conductive-polymer PTC device. Distribution of ceramic particles at: (a) normal operating temperature and (b) trip temperature.
�
406
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
permeability, these microporous separators display a protective
property during cell abuse. For example, if the cell temperature
rises abnormally because of an excessive overcharge, for exam-
ple, the heat generated softens PE and closes the micropores
in the film. This is called separator “shutdown” [50,51]. Once
shutdown occurs, ionic transport between the electrodes is effec-
tively stopped and current ceases to flow [51]. If the separator
can retain mechanical integrity above its shutdown temperature,
it can provide a margin of safety to the device; otherwise, the
electrodes can come into direct contact, react chemically, leading
to thermal runaway. However, it is possible that due to thermal
inertia the temperature can continue to rise even after shutdown.
Under such conditions the separator would melt and short the
electrodes, leading to violent reactions and heat generation. This
phenomenon is called “meltdown” or “breakdown” of the sep-
arator [48]. Therefore, in order to ensure safety of the cell, the
difference between the “shutdown” and “meltdown” tempera-
tures should be as large as possible.
◦
◦
◦
Separators made entirely of high-density polyethylene melt
at 135
C and lose mechanical integrity above this temperature.
However, separators made by laminating layers of polypropy-
lene and polyethylene maintain mechanical integrity at least up
to 165
C, the melting point of polypropylene. It is interesting
to note that although ultrahigh molecular weight polyethylene
melts at 135
C, separators made from this material retain their
mechanical integrity up to at least 180
C as the viscosity of the
material is such that it maintains physical integrity. Shutdown
separators are reliable and lithium-ion battery manufacturers are
increasingly opting for their incorporation in their products.
The most common shutdown separators have high molecu-
lar weight polypropylene blended with super-high molecular
weight polyethylene [45]. Here, the unique shutdown property
of polyethylene is combined favorably with the high mechanical
integrity of polypropylene at elevated temperatures. Because the
shutdown is irreversible, once actuated, these separators leave
the cells permanently damaged.
◦
6. Electrolytes
The key to a safe high-performance lithium-ion cell lies in
the identification of a suitable electrolyte. Lithium is intrinsi-
cally unstable with any commonly known electrolyte. More-
over, lithium battery electrolytes based on alkyl carbonate
solvents are known to react vigorously at elevated temper-
atures with lithiated graphite and delithiated cathodes (e.g.,
LixCoO2 (x < 0.5)) [19,52–54]. At elevated temperatures, the
SEI on the graphite anode gets destroyed, allowing rapid and
direct reaction with the lithiated graphite underneath the pas-
sivating layer. In their delithiated forms, cathodes are highly
oxidizing and enter into exothermic reactions with alkyl car-
bonates, especially at elevated temperatures. Careful calori-
metric studies have thus become mandatory to determine
the safety of electrode–electrolyte combinations. According
to Aurbach et al. [55], commonly used electrolytes such as
LiPF6 in EC–DEC–DMC are only a compromise. They are
flammable and their electrochemical windows are limited to
about 4.5 V. Alternatives to such alkyl carbonate solvents are
Fig. 2. Representation of the variation in the resistance of a PTC device as a
function of temperature.
allows a circuit current insufficient to cause damage but enough
to maintain the device’s trip temperature. The safety device thus
gets latched in its tripped state. It must be pointed out that
conductive-polymer PTC devices allow a small residual leakage
current through the circuit after its tripped state. The resulting
voltage drop across the device can be a concern in certain gad-
gets, especially those that demand precise power requirements.
Conductive-polymer PTC current-limiters still have some
inherent problems. Although they can trip in a few millisec-
onds’ time, their response times are still inferior to those of
fuses. However, they are suitable for applications where a slow-
blow fuse-like characteristic is tolerated. They are also costlier
than common fuses. Moreover, the maximum current and volt-
age they can tolerate are also limited. However, as compared
to their ceramic counterparts, conductive-polymer PTC current
limiters have low normal resistances, which means that they
do not influence the normal operation of the battery. Moreover,
because of their low thermal mass, polymeric PTC devices react
quickly to over-current conditions. Additionally, small surface-
mount polymeric PTC devices can be constructed.
As noted above, an important concern with external safety
mechanisms such as PTC devices is their ability to respond when
hazardous reactions occur at high rates [46]. In order to obviate
this problem, Feng et al. [47] developed an internal self-actuating
thermal cut-off mechanism. Here, the electrodes themselves
would have a PTC effect, an effect that is achieved by coating
the current collector with a suitable PTC material. According to
Feng et al. [47], this would be much more responsive to inter-
nal heat changes than external PTC devices, providing cut-off at
a pre-set activation temperature. Moreover, these internal PTC
devices are reversible, which ensures reusability of the batteries
once the reason for heat build-up is removed.
5. Shutdown separators
Separators for lithium-ion batteries are polyolefin microp-
orous films and are generally uniaxially drawn polyethylene
(PE) and polypropylene (PP), biaxially drawn PE or multi-
axially drawn PP/PE/PP [48,49]. In addition to conventional
characteristics such as good mechanical strength, electrolyte
�
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
407
not on the horizon although alternative salts such as lithium
bis(oxalato)borate, LiBC4O8 (LiBOB) [56], and lithium flu-
oroalkylphosphates (e.g., Li[PF3(C2F5)3]) [57–59] are being
considered in place of LiPF6. Aurbach et al. [55] suggest that
under the circumstances, it is only prudent that additives that
can protect electrode-active materials even at high temperatures
by forming highly protective films on the electrodes be investi-
gated. In fact, new formulations of solvents and salts are unveiled
continually with an eye on safety and performance. A number
of additives are also being investigated to make up for problems
due to protective films at the positive and negative electrodes.
Additives have also been sought to lower electrolyte flammabil-
ity under cell venting. Redox couples that shuttle back and forth
as additives to limit overcharge and additives that produce gas for
activating current interrupter devices have also attracted interest.
6.1. Non-flammable electrolytes
◦
Solvents used in lithium-ion batteries are typically low-
boiling and have flash points around 30
C. Thus, a major danger
from a cell that vents or explodes arises from the flammabil-
ity of the hot electrolyte vapors that are spewed out. Although
identification of a solvent–salt combination that not only pos-
sesses desirable properties for use in batteries but also has the
ability to resist combustion under heat or in the presence of an
external flame may only be a dream, it is possible to develop elec-
trolytes that are not easily flammable [60–67]. Thus, the aim is
to look for “low flammability” or “flame retarding” electrolytes
that do not support continued combustion when the source of
heat, spark or flame is withdrawn. An important consideration
here is that the heat of reaction of the electrolyte with the charged
electrode materials should also be low so that a self-sustaining
combustion reaction does not occur under accidental heating.
Present-day electrolyte formulations are a trade-off between
the electrolyte’s flammability and performance in the cell. The
reduced battery performance is due either to electrochemical
instability (which leads to capacity fading) or increased viscos-
ity of the additive (which affects capacity utilization and power).
Since performance cannot be sacrificed, studies mostly focus on
flame-retardants as the additives or co-solvents in known elec-
trolytes [60–64]. Fluorinated compounds [61] and organophos-
phorus compounds [61,62,68] are among the most investigated
as co-solvents to decrease flammability. For example, trimethyl
phosphate, a popular flame retardant, has been studied for its
electrochemical stability on the positive and negative electrodes
of lithium-ion cells [60–63]. However, it is important to note that
since electrolytes react with the active materials in lithium-ion
batteries, the surface chemistry at the anode and cathode is a
key factor that decides cell performance. Therefore, the design
of new electrolytes must also consider the properties of the SEI
formed with the electrolyte.
6.2. Redox shuttles
Fig. 3. A schematic showing the working of a redox shuttle. Compound R gets
oxidized at the positive electrode to O, which diffuses to the negative electrode
and gets reduced to the original molecule.
positive electrode at potentials slightly higher than the typi-
cal charging plateau. The oxidized forms of these molecules
diffuse to the negative electrode, where they get reduced with-
out side-reactions back to the starting neutral molecules, which
then shuttle back to the positive electrode (Fig. 3). Thus, redox
shuttles shunt the excess charge injected into the cell during
overcharge. In this way, redox shuttles can indefinitely ‘lock’ the
cathode potential at the oxidation potential of neutral molecules
until termination of the charge. In principle, all the Faradaic cur-
rent goes for the reversible reactions, which means that the redox
couple acts as a controlled internal short. A necessary condition
is that both the oxidized and reduced forms of the molecules be
mobile in the electrolyte.
It is possible to visualize scenarios under which the over-
charge current becomes too high for the redox couple to carry,
letting the excess current to delithiate the cathode and causing
irreversible decompositions. To avoid consequent safety hazards
arising under this condition, the current limit that can be shunted
should be maximized by employing large concentrations of the
shuttling molecules [70]. The identification of such a redox
species is fraught with several conditionalities: (i) both the
oxidized and reduced forms of the redox molecule must not
only be inert towards cell constituents, but also have sufficient
thermal stability; (ii) the solubility and diffusion coefficient of
the shuttling molecules in the non-aqueous battery electrolyte
should be high; (iii) the oxidation potential of the redox
couple must be lower than the decomposition potential of the
electrolyte solvents but slightly higher than the overcharge
cut-off voltage; (iv) the shuttle must be electrochemically
reversible and must not enter into side-reactions; and (v) the
reversibility of the couple should last for the entire lifetime
of the cell. A number of soluble redox couples have been
suggested as shuttles for overcharge protection, but they work
only at high charging voltages, which means they actually do
not respond to heat generation in batteries.
Redox shuttles are among the most promising mechanisms
for overcharge protection [69]. The working of redox shuttles,
added to electrolytes, involves electrochemical oxidation at the
The earliest shuttles, employed for 3-V lithium metal
batteries, were based on halides [71,72]. For example, the
iodine/iodide couple can be oxidized at the cathode at 3.20 V
6.2.1. Halide shuttles
�
408
P.G. Balakrishnan et al. / Journal of Power Sources 155 (2006) 401–414
and reduced at the lithium anode. Halide shuttles were, how-
ever, abandoned as the volatility and reactivity of the oxidized
forms (free halogens) rendered such cells impracticable.
6.2.2. Metallocene shuttles
Metallocenes, which form redox pairs, MC/MC+, are among
the earliest chemical shuttles investigated, and were tested in
3-V lithium cells [73–76]. The redox potentials of these couples
can be tuned by varying the substituent groups at the cyclopen-
tadienyl rings [77]. In fact, the potential can be tailored by as
much as 450 mV by varying the number and electron-donating
or electron-withdrawing nature of the substituents. Ferrocene
shuttles have withstood over a hundred “turnovers” in lithium
cells. However, ferrocenes can block ionic paths on the sur-
face of the cathode, which can reduce the power capabilities of
the cell. Moreover, their adsorption on the cathode can result
in capacity loss [74]. The situation is more complex in the
case of lithium-ion cells, for application in which the poten-
tial of the redox couple must be higher than the end-of-charge
electrode potential (say, 4.3 V versus Li+/Li). Additionally, the
SEI-covered graphite electrode in lithium-ion cells does not sup-
port the reduction of MC+, rendering the soluble redox couple
inoperable.
son they are called “shutdown additives” in the battery indus-
try [81,83]. There are two classes of shutdown additives: one
releases gases, which in turn activate a current interrupter device,
while the other undergoes polymerization, thereby blocking ion
transport in the electrolyte. Gas-releasing shutdown additives
include biphenyl [81,84], cyclohexylbenzene [81], pyrocarbon-
ates [81] and phenyl-tert-butyl carbonate [85]. Biphenyl and
other substituted aromatic compounds constitute the polymer-
izable class of shutdown additives [86–88]. It can be deduced
that both the gassing and polymerizable additives are sure-shots
against overcharging. Thus, an approach to a reliable line of
defense against catastrophic failure due to overcharging would
be to incorporate a redox shuttle and a shutdown additive in
the cell such that the activation potential of the latter is higher.
Nevertheless, given the paramount importance of safety, espe-
cially in consumer gadgets and children’s toys, redox chemical
shuttles, which can only provide limited overcharge protection
[79,89] and cannot prevent catastrophic failure as obtained dur-
ing severe overcharging, shutdown protection mechanisms such
as polymerizable additives must be incorporated even at the cost
of termination of useful life of the battery. Some studies have
extended the polymerization reaction into the separator, immo-
bilizing the electrolyte [90].
6.2.3. Aromatic redox shuttles
6.4. Ionic liquids
Redox shuttles based on aromatic compounds have also been
investigated. They include 3-V cell shuttles such as tetracya-
noethylene and tetramethylphenylenediamine [78]. The selec-
tion of 4-V cell shuttles for lithium-ion cells based on LiMn2O4,
LiCoO2 and LiNiO2, however, presents more challenges as only
a handful of substances are amenable to reversible turnover at
potentials around 4.0 V versus Li+/Li. These include complexes
of cerium, iridium, iron or ruthenium with phenanthroline or
bipyridine. Again, their redox potentials can be tuned by varying
the number, nature and position of the substituents on the aro-
matic rings [79]. However, their shunting currents are low due
partly to their limited solubilities in non-aqueous electrolytes
and partly to the low diffusion coefficients resulting from their
large sizes and molecular weights.
According to Adachi et al. [79] anisole-based compounds,
which have high solubility in lithium battery electrolytes, should
make for a better class of redox shuttles. Anisole compounds
with two methoxy groups at 1,2-(ortho-) and 1,4-(para-) posi-
tions display reversibility at the 4-V region [79]. The authors
suggest an empirical structure–property relationship for anisole-
type shuttles, which can help in the design and selection of
more efficient redox shuttles [80]. Adachi et al. [79] conclude
that 4-bromo-1,2-dimethoxybenzene provides the best shunt-
ing performance among 4-V shuttles. Furthermore, anisole-type
shuttles are stable against reduction at carbonaceous anodes.
Other 4-V shuttles reported in the literature include bipyridyl
and biphenyl carbonates as well as difluoroanisoles [81,82].
6.3. Shutdown additives
Some less known additives, also intended for overcharge
protection, terminate cell operation permanently. For this rea-
Several room temperature ionic liquids (molten salts) present
themselves as possible electrolytes in lithium-ion batteries
[91–95]. Not only are they not prone to forming SEI on
the electrodes, they have inherent safety characteristics by
virtue of their thermal stability, non-flammability, non-volatililty
and low heat of reaction with active materials. The non-
flammability is effective in preventing batteries from catching
fire, while non-volatility prevents batteries from bursting. Fur-
thermore, they have favorable electrochemical stability win-
dows for application in lithium-ion cells. One of the central
issues is identification of ionic liquids with sufficient lithium-
ions to allow high flux of lithium-ion through the electrolyte
[96].
Ionic liquids based on the (1-ethyl-3-methylimidazolium)
cation are particularly interesting because of their low viscosi-
ties [97–99], but are not sufficiently stabile towards reduc-
tion in the lithium-ion cell environment [95]. An other-
wise potential electrolyte,
the imidazolium salt, 1-ethyl-3-
methylimidazolium tetrafluoroborate (EMI-BF4), has a reduc-
tion potential of about 1 V versus Li+/Li, which is too high
for lithium battery electrolytes [93,99–102]. However, with the
hope that the reduction potential of the EMI+ cation can be
tailored by incorporation of organic functional groups [100],
Hayashi et al. [103] developed alkylated EMI-BF4 molten
salts, 1-ethyl-2,3,4,5-tetramethylimidazolium tetrafluoroborate
and 1,2-diethyl-3,4(5)-dimethylimidazolium tetrafluoroborate.
Both salts exhibited very little decomposition at 0 V versus
Li+/Li and a wide electrochemical window (up to 5 V ver-
sus Li+/Li). Additionally, the latter electrolyte had a relatively
◦
low melting point of about 20
C and a good specific con-
−1 at 20
◦
ductivity of 1.44 mS cm
C. Quaternary ammonium
�















 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc