ChestX-ray8: Hospital-scale Chest X-ray Database and Benchmarks on
Weakly-Supervised Classification and Localization of Common Thorax Diseases
Xiaosong Wang1, Yifan Peng 2, Le Lu 1, Zhiyong Lu 2, Mohammadhadi Bagheri 1, Ronald M. Summers 1
1Department of Radiology and Imaging Sciences, Clinical Center,
2 National Center for Biotechnology Information, National Library of Medicine,
National Institutes of Health, Bethesda, MD 20892
{xiaosong.wang,yifan.peng,le.lu,luzh,mohammad.bagheri,rms}@nih.gov
Abstract
The chest X-ray is one of the most commonly accessi-
ble radiological examinations for screening and diagnosis
of many lung diseases. A tremendous number of X-ray
imaging studies accompanied by radiological reports are
accumulated and stored in many modern hospitals’ Pic-
ture Archiving and Communication Systems (PACS). On
the other side, it is still an open question how this type
of hospital-size knowledge database containing invaluable
imaging informatics (i.e., loosely labeled) can be used to fa-
cilitate the data-hungry deep learning paradigms in build-
ing truly large-scale high precision computer-aided diagno-
sis (CAD) systems.
In this paper, we present a new chest X-ray database,
namely “ChestX-ray8”, which comprises 108,948 frontal-
view X-ray images of 32,717 unique patients with the text-
mined eight disease image labels (where each image can
have multi-labels), from the associated radiological reports
using natural language processing. Importantly, we demon-
strate that these commonly occurring thoracic diseases can
be detected and even spatially-located via a unified weakly-
supervised multi-label image classification and disease lo-
calization framework, which is validated using our proposed
dataset. Although the initial quantitative results are promis-
ing as reported, deep convolutional neural network based
“reading chest X-rays” (i.e., recognizing and locating the
common disease patterns trained with only image-level la-
bels) remains a strenuous task for fully-automated high pre-
cision CAD systems.
1 Introduction
The rapid and tremendous progress has been evidenced
in a range of computer vision problems via deep learning
and large-scale annotated image datasets [25, 37, 13, 27].
Drastically improved quantitative performances in object
recognition, detection and segmentation are demonstrated in
Figure 1. Eight common thoracic diseases observed in chest X-rays
that validate a challenging task of fully-automated diagnosis.
comparison to previous shallow methodologies built upon
hand-crafted image features. Deep neural network rep-
resentations further make the joint language and vision
learning tasks more feasible to solve, in image captioning
[47, 23, 32, 46, 22], visual question answering [2, 45, 49, 53]
and knowledge-guided transfer learning [4, 33], and so
on. However, the intriguing and strongly observable per-
formance gaps of the current state-of-the-art object detec-
tion and segmentation methods, evaluated between using
PASCAL VOC [13] and employing Microsoft (MS) COCO
[27], demonstrate that there is still significant room for per-
formance improvement when underlying challenges (rep-
resented by different datasets) become greater. For exam-
ple, MS COCO is composed of 80 object categories from
200k images, with 1.2M instances (350k are people) where
every instance is segmented and many instances are small
objects. Comparing to PASCAL VOC of only 20 classes
and 11,530 images containing 27,450 annotated objects with
bounding-boxes (BBox), the top competing object detection
approaches achieve in 0.413 in MS COCO versus 0.884 in
PASCAL VOC under mean Average Precision (mAP).
Deep learning yields similar rises in performance in the
medical image analysis domain for object (often human
anatomical or pathological structures in radiology imaging)
12097
�
detection and segmentation tasks. Recent notable work in-
cludes (but do not limit to) an overview review on the future
promise of deep learning [14] and a collection of important
medical applications on lymph node and interstitial lung dis-
ease detection and classification [36, 42]; cerebral microb-
leed detection [11]; pulmonary nodule detection in CT im-
ages [39]; automated pancreas segmentation [35]; cell im-
age segmentation and tracking [34], predicting spinal radi-
ological scores [20] and extensions of multi-modal imaging
segmentation [29, 16]. The main limitation is that all pro-
posed methods are evaluated on some small-to-middle scale
problems of (at most) several hundred patients. It remains
unclear how well the current deep learning techniques will
scale up to tens of thousands of patient studies.
In the era of deep learning in computer vision, re-
search efforts on building various annotated image datasets
[37, 13, 27, 2, 32, 53, 22, 24] with different characteristics
play indispensably important roles on the better definition
of the forthcoming problems, challenges and subsequently
possible technological progresses. Particularly, here we fo-
cus on the relationship and joint learning of image (chest X-
rays) and text (X-ray reports). The previous representative
image caption generation work [47, 23] utilize Flickr8K,
Flickr30K [51] and MS COCO [27] datasets that hold 8,000,
31,000 and 123,000 images respectively and every image is
annotated by five sentences via Amazon Mechanical Turk
(AMT). The text generally describes annotator’s attention
of objects and activity occurring on an image in a straight-
forward manner. Region-level ImageNet pre-trained con-
volutional neural networks (CNN) based detectors are used
to parse an input image and output a list of attributes or
“visually-grounded high-level concepts” (including objects,
actions, scenes and so on) in [23, 49]. Visual question an-
swering (VQA) requires more detailed parsing and complex
reasoning on the image contents to answer the paired natural
language questions. A new dataset containing 250k natural
images, 760k questions and 10M text answers [2] is pro-
vided to address this new challenge. Additionally, databases
such as “Flickr30k Entities” [32], “Visual7W” [53] and “Vi-
sual Genome” [24, 22] (as detailed as 94,000 images and
4,100,000 region-grounded captions) are introduced to con-
struct and learn the spatially-dense and increasingly diffi-
cult semantic links between textual descriptions and image
regions through the object-level grounding.
Though one could argue that the high-level analogy ex-
ists between image caption generation, visual question an-
swering and imaging based disease diagnosis [41, 40], there
are three factors making truly large-scale medical image
based diagnosis (e.g., involving tens of thousands of pa-
tients) tremendously more formidable. 1, Generic, open-
ended image-level anatomy and pathology labels cannot be
obtained through crowd-sourcing, such as AMT, which is
prohibitively implausible for non-medically trained annota-
tors. Therefore we exploit to mine the per-image (possi-
bly multiple) common thoracic pathology labels from the
image-attached chest X-ray radiological reports using Nat-
ural Language Processing (NLP) techniques. Radiologists
tend to write more abstract and complex logical reasoning
sentences than the plain describing texts in [51, 27]. 2, The
spatial dimensions of an chest X-ray are usually 2000×3000
pixels. Local pathological image regions can show hugely
varying sizes or extents but often very small comparing to
the full image scale. Fig. 1 shows eight illustrative examples
and the actual pathological findings are often significantly
smaller (thus harder to detect). Fully dense annotation of
region-level bounding boxes (for grounding the pathologi-
cal findings) would normally be needed in computer vision
datasets [32, 53, 24] but may be completely nonviable for
the time being. Consequently, we formulate and verify a
weakly-supervised multi-label image classification and dis-
ease localization framework to address this difficulty. 3,
So far, all image captioning and VQA techniques in com-
puter vision strongly depend on the ImageNet pre-trained
deep CNN models which already perform very well in a
large number of object classes and serves a good baseline
for further model fine-tuning. However, this situation does
not apply to the medical image diagnosis domain. Thus we
have to learn the deep image recognition and localization
models while constructing the weakly-labeled medical im-
age database.
To tackle these issues, we propose a new chest X-ray
database, namely “ChestX-ray8”, which comprises 108,948
frontal-view X-ray images of 32,717 (collected from the
year of 1992 to 2015) unique patients with the text-mined
eight common disease labels, mined from the text radi-
ological reports via NLP techniques.
In particular, we
demonstrate that these commonly occurred thoracic dis-
eases can be detected and even spatially-located via a uni-
fied weakly-supervised multi-label image classification and
disease localization formulation. Our initial quantitative re-
sults are promising. However developing fully-automated
deep learning based “reading chest X-rays” systems is still
an arduous journey to be exploited. Details of accessing the
ChestX-ray8 dataset can be found in our website 1.
1.1 Related Work
There have been recent efforts on creating openly avail-
able annotated medical image databases [48, 50, 36, 35]
with the studied patient numbers ranging from a few hun-
dreds to two thousands. Particularly for chest X-rays, the
largest public dataset is OpenI [1] that contains 3,955 ra-
diology reports from the Indiana Network for Patient Care
and 7,470 associated chest x-rays from the hospitals picture
archiving and communication system (PACS). This database
is utilized in [41] as a problem of caption generation but
no quantitative disease detection results are reported. Our
1https://www.cc.nih.gov/drd/summers.html
2098
�
newly proposed chest X-ray database is at least one order
of magnitude larger than OpenI [1] (Refer to Table 1). To
achieve the better clinical relevance, we focus to exploit
the quantitative performance on weakly-supervised multi-
label image classification and disease localization of com-
mon thoracic diseases, in analogy to the intermediate step
of “detecting attributes” in [49] or “visual grounding” for
[32, 53, 22].
2 Construction of Hospital-scale Chest X-ray
Database
In this section, we describe the approach for build-
ing a hospital-scale chest X-ray image database, namely
“ChestX-ray8”, mined from our institute’s PACS system.
First, we short-list eight common thoracic pathology key-
words that are frequently observed and diagnosed, i.e., At-
electasis, Cardiomegaly, Effusion, Infiltration, Mass, Nod-
ule, Pneumonia and Pneumathorax (Fig. 1), based on radi-
ologists’ feedback. Given those 8 text keywords, we search
the PACS system to pull out all the related radiological re-
ports (together with images) as our target corpus. A vari-
ety of Natural Language Processing (NLP) techniques are
adopted for detecting the pathology keywords and removal
of negation and uncertainty. Each radiological report will
be either linked with one or more keywords or marked
with ’Normal’ as the background category. As a result, the
ChestX-ray8 database is composed of 108,948 frontal-view
X-ray images (from 32,717 patients) and each image is la-
beled with one or multiple pathology keywords or “Normal”
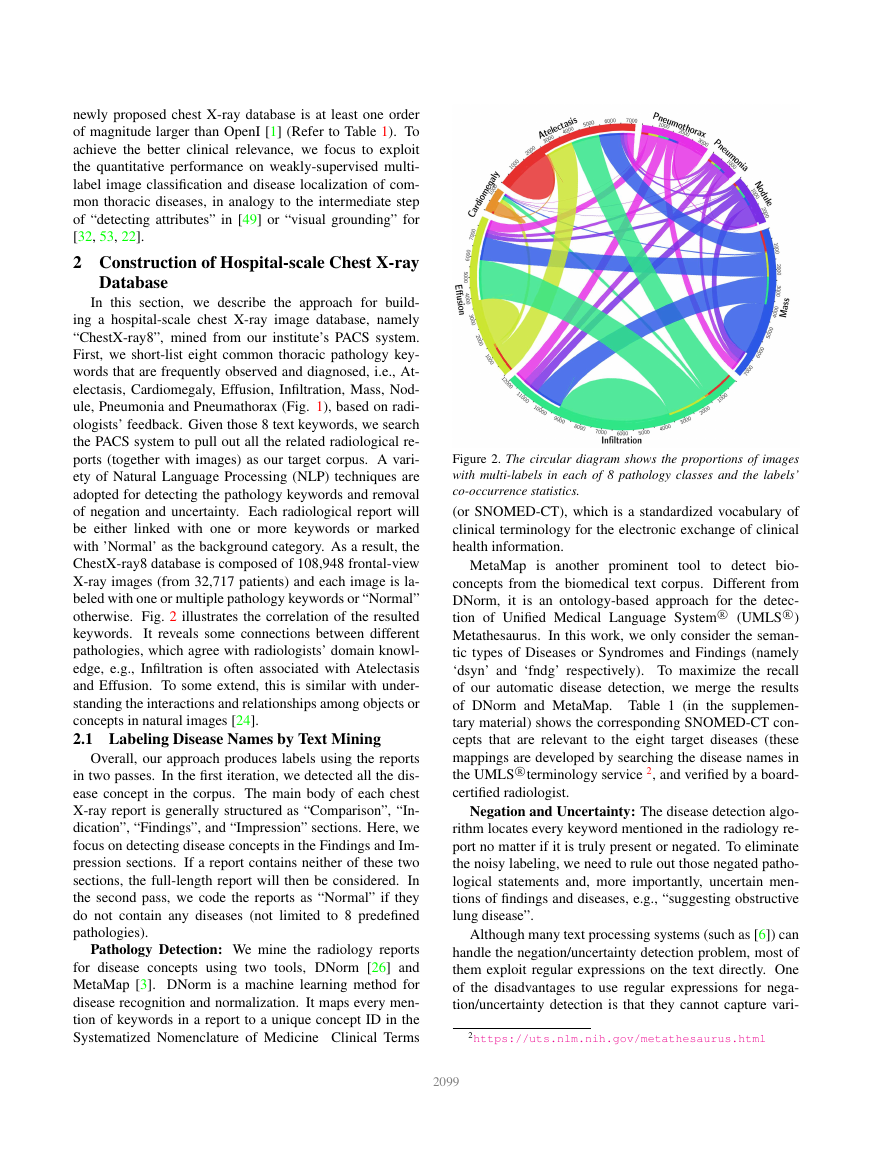
otherwise. Fig. 2 illustrates the correlation of the resulted
keywords.
It reveals some connections between different
pathologies, which agree with radiologists’ domain knowl-
edge, e.g., Infiltration is often associated with Atelectasis
and Effusion. To some extend, this is similar with under-
standing the interactions and relationships among objects or
concepts in natural images [24].
2.1 Labeling Disease Names by Text Mining
Overall, our approach produces labels using the reports
in two passes. In the first iteration, we detected all the dis-
ease concept in the corpus. The main body of each chest
X-ray report is generally structured as “Comparison”, “In-
dication”, “Findings”, and “Impression” sections. Here, we
focus on detecting disease concepts in the Findings and Im-
pression sections. If a report contains neither of these two
sections, the full-length report will then be considered. In
the second pass, we code the reports as “Normal” if they
do not contain any diseases (not limited to 8 predefined
pathologies).
Pathology Detection: We mine the radiology reports
for disease concepts using two tools, DNorm [26] and
MetaMap [3]. DNorm is a machine learning method for
disease recognition and normalization. It maps every men-
tion of keywords in a report to a unique concept ID in the
Systematized Nomenclature of Medicine Clinical Terms
Figure 2. The circular diagram shows the proportions of images
with multi-labels in each of 8 pathology classes and the labels’
co-occurrence statistics.
(or SNOMED-CT), which is a standardized vocabulary of
clinical terminology for the electronic exchange of clinical
health information.
tool
MetaMap is another prominent
to detect bio-
concepts from the biomedical text corpus. Different from
DNorm, it is an ontology-based approach for the detec-
tion of Unified Medical Language System R (UMLS R)
Metathesaurus. In this work, we only consider the seman-
tic types of Diseases or Syndromes and Findings (namely
‘dsyn’ and ‘fndg’ respectively). To maximize the recall
of our automatic disease detection, we merge the results
of DNorm and MetaMap. Table 1 (in the supplemen-
tary material) shows the corresponding SNOMED-CT con-
cepts that are relevant to the eight target diseases (these
mappings are developed by searching the disease names in
the UMLS Rterminology service 2, and verified by a board-
certified radiologist.
Negation and Uncertainty: The disease detection algo-
rithm locates every keyword mentioned in the radiology re-
port no matter if it is truly present or negated. To eliminate
the noisy labeling, we need to rule out those negated patho-
logical statements and, more importantly, uncertain men-
tions of findings and diseases, e.g., “suggesting obstructive
lung disease”.
Although many text processing systems (such as [6]) can
handle the negation/uncertainty detection problem, most of
them exploit regular expressions on the text directly. One
of the disadvantages to use regular expressions for nega-
tion/uncertainty detection is that they cannot capture vari-
2https://uts.nlm.nih.gov/metathesaurus.html
2099
�
prep of
conj or
conj or
... clear of
focal airspace disease , pneumothorax , or pleural effusion
prep of (CCProcessed)
prep of (CCProcessed)
Figure 3. The dependency graph of text: “clear of focal airspace
disease, pneumothorax, or pleural effusion”.
ous syntactic constructions for multiple subjects. For exam-
ple, in the phrase of “clear of A and B”, the regular expres-
sion can capture “A” as a negation but not “B”, particularly
when both “A” and “B” are long and complex noun phrases
(“clear of focal airspace disease, pneumothorax, or pleural
effusion” in Fig. 3).
To overcome this complication, we hand-craft a number
of novel rules of negation/uncertainty defined on the syn-
tactic level in this work. More specifically, we utilize the
syntactic dependency information because it is close to the
semantic relationship between words and thus has become
prevalent in biomedical text processing. We defined our
rules on the dependency graph, by utilizing the dependency
label and direction information between words.
As the first step of preprocessing, we split and tokenize
the reports into sentences using NLTK [5]. Next we parse
each sentence by the Bllip parser [7] using David Mc-
Closkys biomedical model [28]. The syntactic dependen-
cies are then obtained from “CCProcessed” dependencies
output by applying Stanford dependencies converter [8] on
the parse tree. The “CCProcessed” representation propa-
gates conjunct dependencies thus simplifies coordinations.
As a result, we can use fewer rules to match more com-
plex constructions. For an example as shown in Fig. 3, we
could use “clear → prep of → DISEASE” to detect three
negations from the text hneg, focal airspace diseasei, hneg,
pneumothoraxi, and hneg, pleural effusioni.
Furthermore, we label a radiology report as “normal” if
it meets one of the following criteria:
• If there is no disease detected in the report. Note that
here we not only consider 8 diseases of interest in this
paper, but all diseases detected in the reports.
• If the report contains text-mined concepts of “normal”
or “normal size” (CUIs C0205307 and C0332506 in
the SNOMED-CT concepts respectively).
Item #
OpenI Ov. ChestX-ray8
Ov.
Report
Annotations
Atelectasis
Cardiomegaly
Effusion
Infiltration
Mass
Nodule
Pneumonia
Pneumothorax
Normal
2,435
2,435
315
345
153
60
15
106
40
22
1,379
-
-
122
100
94
45
4
18
15
11
0
108,948
-
5,789
1,010
6,331
10,317
6,046
1,971
1,062
2,793
84,312
-
-
3,286
475
4,017
4,698
3,432
1,041
703
1,403
0
Table 1. Total number (#) and # of Overlap (Ov.) of the corpus in
both OpenI and ChestX-ray8 datasets.
Disease
Atelectasis
Cardiomegaly
Effusion
Infiltration
Mass
Nodule
Normal
Pneumonia
Pneumothorax
Total
MetaMap
P / R /
F
Our Method
P / R /
F
0.95 / 0.95 / 0.95
0.99 / 0.83 / 0.90
0.74 / 0.90 / 0.81
0.25 / 0.98 / 0.39
0.59 / 0.67 / 0.62
0.95 / 0.65 / 0.77
0.93 / 0.90 / 0.91
0.58 / 0.93 / 0.71
0.32 / 0.82 / 0.46
0.84 / 0.88 / 0.86
0.99 / 0.85 / 0.91
1.00 / 0.79 / 0.88
0.93 / 0.82 / 0.87
0.74 / 0.87 / 0.80
0.75 / 0.40 / 0.52
0.96 / 0.62 / 0.75
0.87 / 0.99 / 0.93
0.66 / 0.93 / 0.77
0.90 / 0.82 / 0.86
0.90 / 0.91 / 0.90
Table 2. Evaluation of image labeling results on OpenI dataset.
Performance is reported using P, R, F1-score.
the gold standard for evaluating our method. Table 1 sum-
marizes the statistics of the subset of OpenI [1, 19] reports.
Table 2 shows the results of our method using OpenI, mea-
sured in precision (P), recall (R), and F1-score. Higher pre-
cision of 0.90, higher recall of 0.91, and higher F1-score
of 0.90 are achieved compared to the existing MetaMap ap-
proach (with NegEx enabled). For all diseases, our method
obtains higher precisions, particularly in “pneumothorax”
(0.90 vs. 0.32) and “infiltration” (0.74 vs. 0.25). This in-
dicates that the usage of negation and uncertainty detection
on syntactic level successfully removes false positive cases.
More importantly, the higher precisions meet our expecta-
tion to generate a Chest X-ray corpus with accurate seman-
tic labels, to lay a solid foundation for the later processes.
2.2 Quality Control on Disease Labeling
2.3 Processing Chest X-ray Images
To validate our method, we perform the following exper-
iments. Given the fact that no gold-standard labels exist for
our dataset, we resort to some existing annotated corpora as
an alternative. Using the OpenI API [1], we retrieve a total
of 3,851 unique radiology reports where each OpenI report
is assigned with its key findings/disease names by human
annotators [9]. Given our focus on the eight diseases, a sub-
set of OpenI reports and their human annotations are used as
Comparing to the popular ImageNet classification prob-
lem, significantly smaller spatial extents of many diseases
inside the typical X-ray image dimensions of 3000 × 2000
pixels impose challenges in both the capacity of comput-
ing hardware and the design of deep learning paradigm. In
ChestX-ray8, X-rays images are directly extracted from the
DICOM file and resized as 1024×1024 bitmap images with-
out significantly losing the detail contents, compared with
2100
�
image sizes of 512 × 512 in OpenI dataset. Their intensity
ranges are rescaled using the default window settings stored
in the DICOM header files.
2.4 Bounding Box for Pathologies
As part of the ChestX-ray8 database, a small number
of images with pathology are provided with hand labeled
bounding boxes (B-Boxes), which can be used as the ground
truth to evaluate the disease localization performance. Fur-
thermore, it could also be adopted for one/low-shot learn-
ing setup [15], in which only one or several samples are
needed to initialize the learning and the system will then
evolve by itself with more unlabeled data. We leave this as
future work.
In our labeling process, we first select 200 instances for
each pathology (1,600 instances total), consisting of 983
images. Given an image and a disease keyword, a board-
certified radiologist identified only the corresponding dis-
ease instance in the image and labeled it with a B-Box. The
B-Box is then outputted as an XML file. If one image con-
tains multiple disease instances, each disease instance is la-
beled separately and stored into individual XML files. As
an application of the proposed ChestX-ray8 database and
benchmarking, we will demonstrate the detection and local-
ization of thoracic diseases in the following.
3 Common Thoracic Disease Detection and
Localization
Reading and diagnosing Chest X-ray images may be an
entry-level task for radiologists but, in fact it is a complex
reasoning problem which often requires careful observation
and good knowledge of anatomical principles, physiology
and pathology. Such factors increase the difficulty of de-
veloping a consistent and automated technique for reading
chest X-ray images while simultaneously considering all
common thoracic diseases.
As the main application of ChestX-ray8 dataset, we
present a unified weakly-supervised multi-label image clas-
sification and pathology localization framework, which can
detect
the presence of multiple pathologies and subse-
quently generate bounding boxes around the corresponding
pathologies. In details, we tailor Deep Convolutional Neural
Network (DCNN) architectures for weakly-supervised ob-
ject localization, by considering large image capacity, vari-
ous multi-label CNN losses and different pooling strategies.
3.1 Unified DCNN Framework
Our goal is to first detect if one or multiple pathologies
are presented in each X-ray image and later we can lo-
cate them using the activation and weights extracted from
the network. We tackle this problem by training a multi-
label DCNN classification model. Fig. 4 illustrates the
DCNN architecture we adapted, with similarity to sev-
eral previous weakly-supervised object localization meth-
ods [30, 52, 12, 18]. As shown in Fig. 4, we perform
the network surgery on the pre-trained models (using Im-
ageNet [10, 38]), e.g., AlexNet [25], GoogLeNet [44],
VGGNet-16 [43] and ResNet-50 [17], by leaving out the
fully-connected layers and the final classification layers. In-
stead we insert a transition layer, a global pooling layer, a
prediction layer and a loss layer in the end (after the last con-
volutional layer). In a similar fashion as described in [52],
a combination of deep activations from transition layer (a
set of spatial image features) and the weights of prediction
inner-product layer (trained feature weighting) can enable
us to find the plausible spatial locations of diseases.
Multi-label Setup: There are several options of image-
label representation and the choices of multi-label classi-
fication loss functions. Here, we define a 8-dimensional
label vector y = [y1, ..., yc, ..., yC], yc ∈ {0, 1}, C = 8
for each image. yc indicates the presence with respect to
according pathology in the image while a all-zero vector
[0, 0, 0, 0, 0, 0, 0, 0] represents the status of “Normal” (no
pathology is found in the scope of any of 8 disease cate-
gories as listed). This definition transits the multi-label clas-
sification problem into a regression-like loss setting.
Transition Layer: Due to the large variety of pre-trained
DCNN architectures we adopt, a transition layer is usu-
ally required to transform the activations from previous lay-
ers into a uniform dimension of output, S × S × D, S ∈
{8, 16, 32}. D represents the dimension of features at spa-
tial location (i, j), i, j ∈ {1, ..., S}, which can be varied in
different model settings, e.g., D = 1024 for GoogLeNet and
D = 2048 for ResNet. The transition layer helps pass down
the weights from pre-trained DCNN models in a standard
form, which is critical for using this layers’ activations to
further generate the heatmap in pathology localization step.
Multi-label Classification Loss Layer: We first experi-
ment 3 standard loss functions for the regression task instead
of using the softmax loss for traditional multi-class classifi-
cation model, i.e., Hinge Loss (HL), Euclidean Loss (EL)
and Cross Entropy Loss (CEL). However, we find that the
model has difficulty learning positive instances (images with
pathologies) and the image labels are rather sparse, mean-
ing there are extensively more ‘0’s than ‘1’s. This is due to
our one-hot-like image labeling strategy and the unbalanced
numbers of pathology and “Normal” classes. Therefore, we
introduce the positive/negative balancing factor βP , βN to
enforce the learning of positive examples. For example, the
weighted CEL (W-CEL) is defined as follows,
LW –CEL(f (~x), ~y) =
βP X
− ln(f (xc)) + βN X
yc=1
yc=0
− ln(1 − f (xc)),
(1)
where βP is set to |P |+|N |
. |P |
and |N | are the total number of ‘1’s and ‘0’s in a batch of
image labels.
|P | while βN is set to |P |+|N |
|N |
2101
�
Figure 4. The overall flow-chart of our unified DCNN framework and disease localization process.
3.2 Weakly-Supervised Pathology Localization
Global Pooling Layer and Prediction Layer: In our
multi-label image classification network, the global pool-
ing and the predication layer are designed not only to be
part of the DCNN for classification but also to generate the
likelihood map of pathologies, namely a heatmap. The lo-
cation with a peak in the heatmap generally corresponds to
the presence of disease pattern with a high probability. The
upper part of Fig. 4 demonstrates the process of producing
this heatmap. By performing a global pooling after the tran-
sition layer, the weights learned in the prediction layer can
function as the weights of spatial maps from the transition
layer. Therefore, we can produce weighted spatial activation
maps for each disease class (with a size of S × S × C) by
multiplying the activation from transition layer (with a size
of S × S × D) and the weights of prediction layer (with a
size of D × C).
The pooling layer plays an important role that chooses
what information to be passed down. Besides the conven-
tional max pooling and average pooling, we also utilize the
Log-Sum-Exp (LSE) pooling proposed in [31]. The LSE
pooled value xp is defined as
xp =
1
r
· log
1
S
· X
(i,j)∈S
exp(r · xij)
,
(2)
where xij is the activation value at (i, j), (i, j) is one loca-
tion in the pooling region S, and S = s × s is the total num-
ber of locations in S. By controlling the hyper-parameter
r, the pooled value ranges from the maximum in S (when
r → ∞) to average (r → 0). It serves as an adjustable op-
tion between max pooling and average pooling. Since the
LSE function suffers from overflow/underflow problems,
the following equivalent is used while implementing the
LSE pooling layer in our own DCNN architecture,
xp = x∗ +
1
r
· log
1
S
· X
(i,j)∈S
exp(r · (xij − x∗)
,
(3)
where x∗ = max{|xij|, (i, j) ∈ S}.
Bounding Box Generation: The heatmap produced
from our multi-label classification framework indicates the
approximate spatial location of one particular thoracic dis-
ease class each time. Due to the simplicity of intensity dis-
tributions in these resulting heatmaps, applying an ad-hoc
thresholding based B-Box generation method for this task is
found to be sufficient. The intensities in heatmaps are first
normalized to [0, 255] and then thresholded by {60, 180} in-
dividually. Finally, B-Boxes are generated to cover the iso-
lated regions in the resulting binary maps.
4 Experiments
Data: We evaluate and validate the unified disease clas-
sification and localization framework using the proposed
ChestX-ray8 database. In total, 108,948 frontal-view X-ray
images are in the database, of which 24,636 images contain
one or more pathologies. The remaining 84,312 images are
normal cases. For the pathology classification and localiza-
tion task, we randomly shuffled the entire dataset into three
2102
�
subgroups for CNN fine-tuning via Stochastic Gradient De-
scent (SGD): i.e. training (70%), validation (10%) and test-
ing (20%). We only report the 8 thoracic disease recognition
performance on the testing set in our experiments. Further-
more, for the 983 images with 1,600 annotated B-Boxes of
pathologies, these boxes are only used as the ground truth to
evaluate the disease localization accuracy in testing (not for
training purpose).
CNN Setting: Our multi-label CNN architecture is im-
plemented using Caffe framework [21]. The ImageNet
pre-trained models, i.e., AlexNet [25], GoogLeNet [44],
VGGNet-16 [43] and ResNet-50 [17] are obtained from the
Caffe model zoo. Our unified DCNN takes the weights from
those models and only the transition layers and prediction
layers are trained from scratch.
Due to the large image size and the limit of GPU mem-
ory, it is necessary to reduce the image batch size to load
the entire model and keep activations in GPU while we in-
crease the iter size to accumulate the gradients for more it-
erations. The combination of both may vary in different
CNN models but we set batch size × iter size = 80 as
a constant. Furthermore, the total training iterations are cus-
tomized for different CNN models to prevent over-fitting.
More complex models like ResNet-50 actually take less it-
erations (e.g., 10000 iterations) to reach the convergence.
The DCNN models are trained using a Dev-Box linux server
with 4 Titan X GPUs.
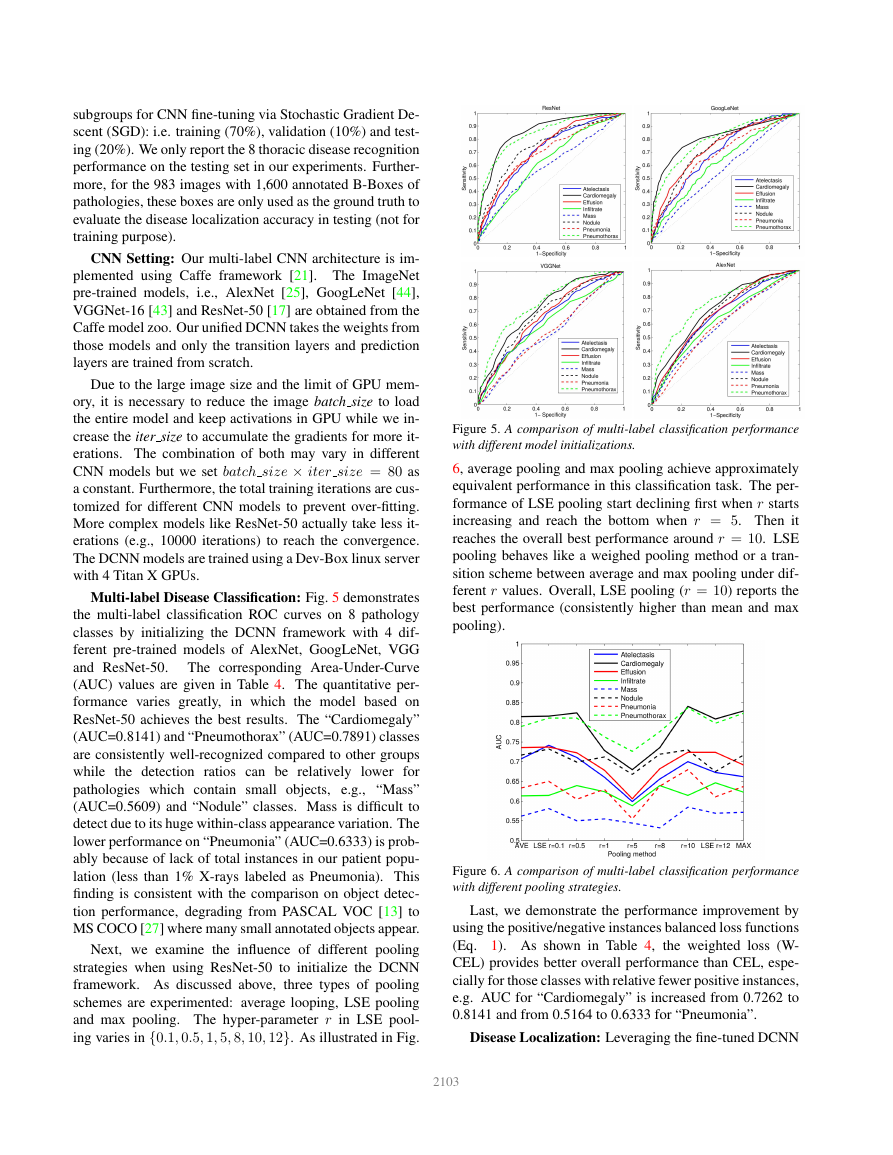
Multi-label Disease Classification: Fig. 5 demonstrates
the multi-label classification ROC curves on 8 pathology
classes by initializing the DCNN framework with 4 dif-
ferent pre-trained models of AlexNet, GoogLeNet, VGG
and ResNet-50.
The corresponding Area-Under-Curve
(AUC) values are given in Table 4. The quantitative per-
formance varies greatly,
in which the model based on
ResNet-50 achieves the best results. The “Cardiomegaly”
(AUC=0.8141) and “Pneumothorax” (AUC=0.7891) classes
are consistently well-recognized compared to other groups
while the detection ratios can be relatively lower for
pathologies which contain small objects, e.g., “Mass”
(AUC=0.5609) and “Nodule” classes. Mass is difficult to
detect due to its huge within-class appearance variation. The
lower performance on “Pneumonia” (AUC=0.6333) is prob-
ably because of lack of total instances in our patient popu-
lation (less than 1% X-rays labeled as Pneumonia). This
finding is consistent with the comparison on object detec-
tion performance, degrading from PASCAL VOC [13] to
MS COCO [27] where many small annotated objects appear.
Next, we examine the influence of different pooling
strategies when using ResNet-50 to initialize the DCNN
framework. As discussed above, three types of pooling
schemes are experimented: average looping, LSE pooling
and max pooling. The hyper-parameter r in LSE pool-
ing varies in {0.1, 0.5, 1, 5, 8, 10, 12}. As illustrated in Fig.
Figure 5. A comparison of multi-label classification performance
with different model initializations.
6, average pooling and max pooling achieve approximately
equivalent performance in this classification task. The per-
formance of LSE pooling start declining first when r starts
increasing and reach the bottom when r = 5. Then it
reaches the overall best performance around r = 10. LSE
pooling behaves like a weighed pooling method or a tran-
sition scheme between average and max pooling under dif-
ferent r values. Overall, LSE pooling (r = 10) reports the
best performance (consistently higher than mean and max
pooling).
Figure 6. A comparison of multi-label classification performance
with different pooling strategies.
Last, we demonstrate the performance improvement by
using the positive/negative instances balanced loss functions
(Eq. 1). As shown in Table 4, the weighted loss (W-
CEL) provides better overall performance than CEL, espe-
cially for those classes with relative fewer positive instances,
e.g. AUC for “Cardiomegaly” is increased from 0.7262 to
0.8141 and from 0.5164 to 0.6333 for “Pneumonia”.
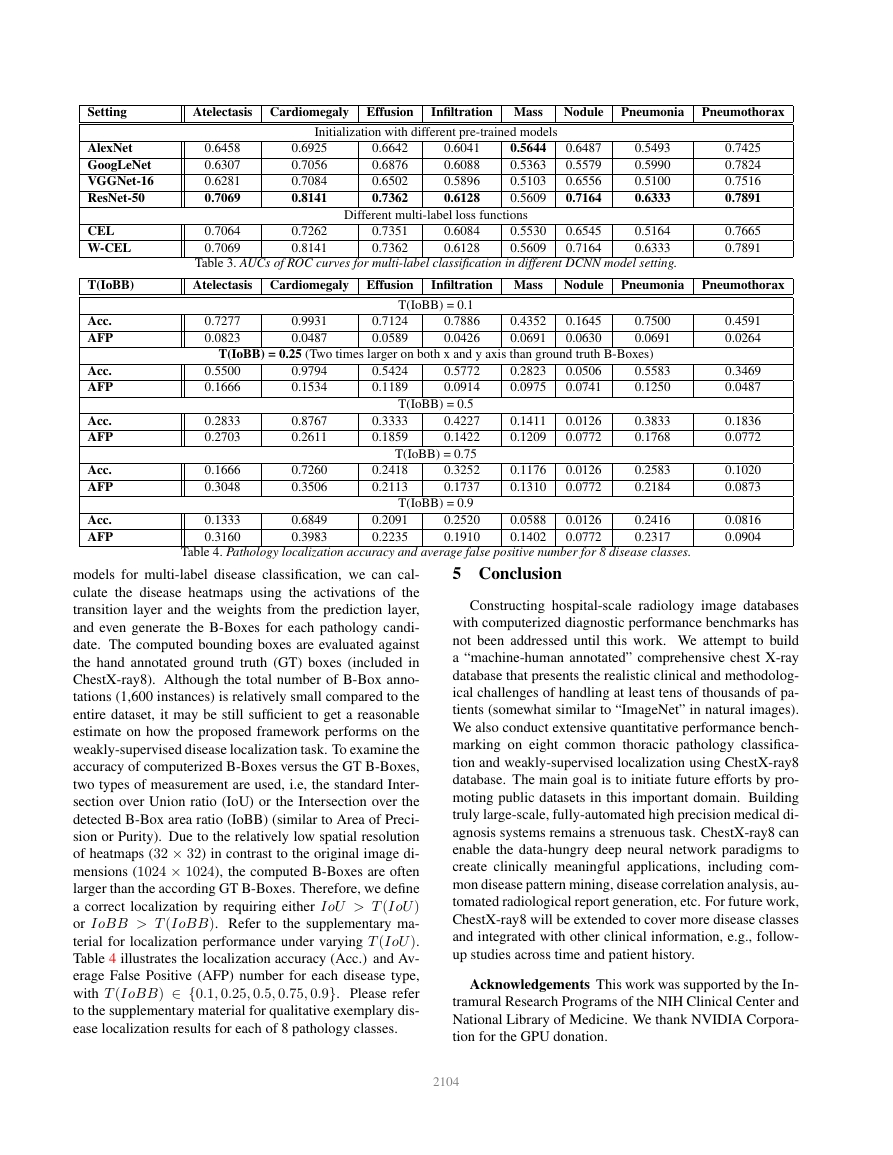
Disease Localization: Leveraging the fine-tuned DCNN
2103
�
Setting
Atelectasis Cardiomegaly Effusion
Infiltration Mass
Nodule
Pneumonia
Pneumothorax
AlexNet
GoogLeNet
VGGNet-16
ResNet-50
CEL
W-CEL
0.6458
0.6307
0.6281
0.7069
0.7064
0.7069
Initialization with different pre-trained models
0.6925
0.7056
0.7084
0.8141
0.7262
0.8141
0.6642
0.6876
0.6502
0.7362
0.6041
0.6088
0.5896
0.6128
0.5644
0.5363
0.5103
0.5609
0.6487
0.5579
0.6556
0.7164
Different multi-label loss functions
0.7351
0.7362
0.6084
0.6128
0.5530
0.5609
0.6545
0.7164
0.5493
0.5990
0.5100
0.6333
0.5164
0.6333
0.7425
0.7824
0.7516
0.7891
0.7665
0.7891
Table 3. AUCs of ROC curves for multi-label classification in different DCNN model setting.
T(IoBB)
Atelectasis Cardiomegaly Effusion
Infiltration Mass
Nodule
Pneumonia
Pneumothorax
Acc.
AFP
Acc.
AFP
Acc.
AFP
Acc.
AFP
Acc.
AFP
T(IoBB) = 0.1
0.7277
0.0823
0.9931
0.0487
0.7124
0.0589
0.7886
0.0426
0.4352
0.0691
0.1645
0.0630
0.7500
0.0691
T(IoBB) = 0.25 (Two times larger on both x and y axis than ground truth B-Boxes)
0.5500
0.1666
0.2833
0.2703
0.1666
0.3048
0.1333
0.3160
0.9794
0.1534
0.8767
0.2611
0.7260
0.3506
0.6849
0.3983
0.5424
0.1189
0.5772
0.0914
0.2823
0.0975
0.0506
0.0741
T(IoBB) = 0.5
0.3333
0.1859
0.2418
0.2113
0.4227
0.1422
T(IoBB) = 0.75
0.3252
0.1737
0.1411
0.1209
0.0126
0.0772
0.1176
0.1310
0.0126
0.0772
T(IoBB) = 0.9
0.2091
0.2235
0.2520
0.1910
0.0588
0.1402
0.0126
0.0772
0.5583
0.1250
0.3833
0.1768
0.2583
0.2184
0.2416
0.2317
0.4591
0.0264
0.3469
0.0487
0.1836
0.0772
0.1020
0.0873
0.0816
0.0904
Table 4. Pathology localization accuracy and average false positive number for 8 disease classes.
models for multi-label disease classification, we can cal-
culate the disease heatmaps using the activations of the
transition layer and the weights from the prediction layer,
and even generate the B-Boxes for each pathology candi-
date. The computed bounding boxes are evaluated against
the hand annotated ground truth (GT) boxes (included in
ChestX-ray8). Although the total number of B-Box anno-
tations (1,600 instances) is relatively small compared to the
entire dataset, it may be still sufficient to get a reasonable
estimate on how the proposed framework performs on the
weakly-supervised disease localization task. To examine the
accuracy of computerized B-Boxes versus the GT B-Boxes,
two types of measurement are used, i.e, the standard Inter-
section over Union ratio (IoU) or the Intersection over the
detected B-Box area ratio (IoBB) (similar to Area of Preci-
sion or Purity). Due to the relatively low spatial resolution
of heatmaps (32 × 32) in contrast to the original image di-
mensions (1024 × 1024), the computed B-Boxes are often
larger than the according GT B-Boxes. Therefore, we define
a correct localization by requiring either IoU > T (IoU )
or IoBB > T (IoBB). Refer to the supplementary ma-
terial for localization performance under varying T (IoU ).
Table 4 illustrates the localization accuracy (Acc.) and Av-
erage False Positive (AFP) number for each disease type,
with T (IoBB) ∈ {0.1, 0.25, 0.5, 0.75, 0.9}. Please refer
to the supplementary material for qualitative exemplary dis-
ease localization results for each of 8 pathology classes.
5 Conclusion
Constructing hospital-scale radiology image databases
with computerized diagnostic performance benchmarks has
not been addressed until this work. We attempt to build
a “machine-human annotated” comprehensive chest X-ray
database that presents the realistic clinical and methodolog-
ical challenges of handling at least tens of thousands of pa-
tients (somewhat similar to “ImageNet” in natural images).
We also conduct extensive quantitative performance bench-
marking on eight common thoracic pathology classifica-
tion and weakly-supervised localization using ChestX-ray8
database. The main goal is to initiate future efforts by pro-
moting public datasets in this important domain. Building
truly large-scale, fully-automated high precision medical di-
agnosis systems remains a strenuous task. ChestX-ray8 can
enable the data-hungry deep neural network paradigms to
create clinically meaningful applications, including com-
mon disease pattern mining, disease correlation analysis, au-
tomated radiological report generation, etc. For future work,
ChestX-ray8 will be extended to cover more disease classes
and integrated with other clinical information, e.g., follow-
up studies across time and patient history.
Acknowledgements This work was supported by the In-
tramural Research Programs of the NIH Clinical Center and
National Library of Medicine. We thank NVIDIA Corpora-
tion for the GPU donation.
2104
�













 2023年江西萍乡中考道德与法治真题及答案.doc
2023年江西萍乡中考道德与法治真题及答案.doc 2012年重庆南川中考生物真题及答案.doc
2012年重庆南川中考生物真题及答案.doc 2013年江西师范大学地理学综合及文艺理论基础考研真题.doc
2013年江西师范大学地理学综合及文艺理论基础考研真题.doc 2020年四川甘孜小升初语文真题及答案I卷.doc
2020年四川甘孜小升初语文真题及答案I卷.doc 2020年注册岩土工程师专业基础考试真题及答案.doc
2020年注册岩土工程师专业基础考试真题及答案.doc 2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc
2023-2024学年福建省厦门市九年级上学期数学月考试题及答案.doc 2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc
2021-2022学年辽宁省沈阳市大东区九年级上学期语文期末试题及答案.doc 2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc
2022-2023学年北京东城区初三第一学期物理期末试卷及答案.doc 2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc
2018上半年江西教师资格初中地理学科知识与教学能力真题及答案.doc 2012年河北国家公务员申论考试真题及答案-省级.doc
2012年河北国家公务员申论考试真题及答案-省级.doc 2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc
2020-2021学年江苏省扬州市江都区邵樊片九年级上学期数学第一次质量检测试题及答案.doc 2022下半年黑龙江教师资格证中学综合素质真题及答案.doc
2022下半年黑龙江教师资格证中学综合素质真题及答案.doc